The Influence of Formulation and Processing on the Retention of B Vitamins in Asian Noodles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nissin Foods Announces Sponsorship for Tennis Star in the Making Coleman Wong Supports the 16-Year-Old Young Athlete to Achieve His Junior Grand Slam Dream
[For Immediate Release] (Incorporated in Hong Kong with limited liability) Stock code: 1475 Nissin Foods announces sponsorship for tennis star in the making Coleman Wong Supports the 16-year-old young athlete to achieve his Junior Grand Slam dream Nissin Foods is committed to support more budding athletes to excel globally Photo 1: Mr. Kiyotaka Ando, Executive Director, Chairman of the Board and Chief Executive Officer of Nissin Foods, speaks highly of Coleman Wong and expects him to vie for more wins in international tournaments. (Hong Kong, 29 June 2020) Nissin Foods Company Limited (“Nissin Foods” or the “Company”, and together with its subsidiaries, the “Group”; Stock code: 1475) today announces that the company will give a 2-year sponsorship in 2020-2021 for Coleman Wong Chak-lam, a sixteen-year-old Hong Kong’s most promising young tennis player, enabling Coleman to vie for more honours in international tournaments. The city’s largest instant noodle and renowned food company has teamed up with the Hong Kong Tennis Association (HKTA) as the official Youth Development Partner for years, and the latest sponsorship for Coleman Wong indicates the brand has again offered its support to an individual tennis player. In pursuit of the founder Mr. Momofuku Ando’s belief that “eating and sports as the two axles of health”, Nissin Foods is an active sponsor of local sports activities in particular the tennis junior development. Nissin Foods has been supporting the local youth tennis tournaments hosted by the Hong Kong Tennis Association since 2015, and is the current sponsor of HKTA’s 3 major tournaments - The Nissin Hong Kong National Junior Tennis Championships, Nissin Cup Noodles Hong Kong Junior Tennis Series, and Nissin Demae Iccho Hong Kong Junior Nissin Foods announces sponsorship for tennis star in the making Coleman Wong Tennis Novice Competition. -

I N V E S T O R S ' G U I
INVESTORS’GUIDE Consolidated Results for the First Half of the Fiscal Year Ending March 31, 2012 (FY 2012) October 27, 2011 Ticker Code 2897 URL http://www.nissinfoods-holdings.co.jp/ Contents Slide NO. Page 3. Business Environments in First Half of FY2012 2 4. Overview of Consolidated Results for First Half of FY2012 2 5. Business Performance by Quarter 3 6. Performance by Reportable Segment 3 7. Breakdown of Operating Income (Consolidated) 4 8. Ordinary Income, Net Income (Consolidated) 4 10. Japan Business – NISSIN FOOD PRODUCTS CO., LTD. 5 New Generations of Instant Noodles 11. 6 Made Possible by Technical Innovation 12. Features of Hybrid Noodles 6 13. Cup Noodle 40th Anniversary Campaign 7 14. Cup Noodle Gohan Seafood Launched Nationwide! 7 Average Price of Mainstay Products at Mass Merchandise Stores 15. 8 (Jan. 2008 to Aug. 2011) 16. Instant Noodle Business Japan – MYOJO FOODS CO., LTD. 8 18. – NISSIN CHILLED FOODS, NISSIN FROZEN FOODS 9 19. Cereal, Beverage Business – NISSIN CISCO, NISSIN YORK 10 21. Overseas Segment Information 11 22. The Americas Segment (Jan. to June 2011) 11 23. China Segment (Jan. to June 2011) 12 25. Forecasts for the Fiscal Year Ending March 2012 (Consolidated) 13 28. Overview of CUPNOODLES MUSEUM 14 A.Supplementary Data A-1. Results for the First Half of FY2012 16 A-2. Results and Forecasts by Segments and Regions 17 A-3. Forecasts for Consolidated Results for FY2012 18 A-4. Exchange Rates for First Half of FY2012 18 A-5. Equity in Earnings of Affiliates 18 A-6. Effect of Retirement Benefit Expenses since FY2008 18 A-7. -

Shifting Perceptions of Instant Ramen in Japan During the High-Growth Era, 1958–1973
IJAPS, Vol. 8, No. 2 (July 2012), 13–31 SHIFTING PERCEPTIONS OF INSTANT RAMEN IN JAPAN DURING THE HIGH-GROWTH ERA, 1958–1973 George Solt1 New York University, United States email: [email protected] ABSTRACT Instant ramen attained national prominence in Japan beginning in 1958 with the release of the first nationally advertised brand, Chikin Ramen, produced and sold by Momofuku Ando's Sanshī Shokuhin, later to be renamed the Nissin Foods Corporation. From the time of its release, instant ramen became one of the most widely advertised products in Japan. The industry, led by Nissin, was exceptionally successful in utilising marketing campaigns to capitalise on social transformations. The advertisements of the Nissin Foods Corporation are particularly useful indicators of shifts in social organisation, reflecting the transformation of norms and sensibilities occurring in Japan during the fifteen years following the introduction of the emblematic food of convenience. Nissin Foods Corporation reinvented its product and shifted advertising emphasis frequently to accommodate the changing milieu with respect to convenience foods. Initially marketed as a healthy meal full of essential vitamins and nutrients that provided an alternative to cooking for busy housewives, instant ramen quickly became a defining product symbolic of postwar youth culture in the 1960s. By tracing the shifts in instant ramen advertising from the earliest ads in newspapers to later spots on television, the essay will examine the evolving form and content of instant noodle advertising in Japan to illuminate the connections between popular food trends and larger social and political changes related to family organisation, nutritional science and projections of national identity. -

Nissin Foods Cooks up a Secure Future for the Company
Case study Hong Kong | Manufacturing Nissin Foods cooks up a secure future for the company Client profile Summary Nissin Foods Company is a spin-off of In order to ensure that their IT infrastructure remained secure, Nissin Foods engaged with the instant noodle operations Nissin us to conduct an assessment of their IT security maturity. The comprehensive assessment, Foods in Japan and a leading food specifically for their network and web applications, provides them with the insights needed and beverage manufacturer in Hong to develop a cybersecurity transformation roadmap, protecting them against current and Kong and China. future threats. As the largest instant noodle company in Hong Kong, their focus Vision is on the manufacturing and sale Security critical for the future of IT infrastructure of premium instant noodles, frozen As a company with a prominent public profile, Nissin Foods in Hong Kong was acutely foods, beverages and snacks. aware of the threat that cybercriminals posed. Technology plays a vital role in their business and is a key enabler of their future business vision, providing the foundation for a flexible and agile organization. Currently they operate ‘ Using NTT’s their IT infrastructure at their own manufacturing facilities in Hong Kong and China, and expertise we now are working on optimizing it by migrating to the cloud. have a clear view of They were looking for a partner who would be able to help them assess their current our current security security maturity level and develop a roadmap to ensure they were able to meet any future security challenges. We were selected not only because of our expertise in security maturity and a consulting, but also of our capability to provide end-to-end services including managed blueprint for the security services and IT infrastructure enhancements after the security assessment. -

India: Food Processing Ingredients
THIS REPORT CONTAINS ASSESSMENTS OF COMMODITY AND TRADE ISSUES MADE BY USDA STAFF AND NOT NECESSARILY STATEMENTS OF OFFICIAL U.S. GOVERNMENT POLICY Required Report - public distribution Date: 12/29/2016 GAIN Report Number: IN6166 India Food Processing Ingredients India’s food processing sector is poised for growth in response to changing demographics, evolving preferences for branded items, a modernizing retail sector, growing consumer acceptance of processed foods, and government advocacy to develop food manufacturing. Approved By: Adam Branson Prepared By: Dhruv Sood and Radha Mani Report Highlights: India’s food processing sector is poised for growth in response to changing demographics, evolving preferences for branded items, a modernizing retail sector, growing consumer acceptance of processed foods, and government advocacy to develop food manufacturing. Packaged food sales almost doubled between 2011 and 2015 to $38 billion and there is opportunity for further growth. Imports of non- standardized foods and ingredients remain a challenge. Post: New Delhi Executive Summary: Section I – Market Summary Increasing urbanization, lifestyle changes, greater affluence, and increased rates of women working outside of the home are driving demand for processed foods. According to the Ministry of Food Processing, the food processing sector accounts for 1.7 percent of gross domestic product and is valued at $25 billion (based on a revised series). According to the latest Annual Survey of Industries, there are 37,175 registered food processing units in the country that have employed approximately 1.7 million people. As per an assessment of the extent of food processing in various food sub-sectors done in 2014 by the Institute of Economic Growth on behalf of the Ministry of Agriculture, the average extent of processing of agro-products in 2010-11 was 6.76 percent. -
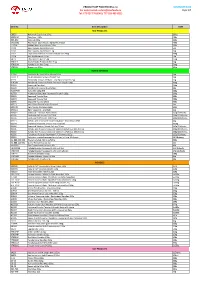
NOVEMBER 2020 Page
PRODUCT LIST TAKO FOODS s.r.o NOVEMBER 2020 For orders email: [email protected] Page 1/7 Tel: 773 027 776 (ENG), 777 926 495 (CZE) Item No. Item Description UoM RICE PRODUCTS 08930 Hakumaki Sushi rice 10 kg 10 kg 303013A Rice flour 400g 400g 839-840 Shiokoji 220g 220g CH16032 Rice Paper 22cm Round Spring-Roll 400g 400g J1371A Malted Rice - Kome Kouji 200g 200g J3100 Rice Toyama Koshihikari 5kg 5kg J3101 Rice Toyama Koshihikari 1 kg 1kg J3102 Toyama Koshihikari Funwari Gohan rice 200g 200g J3104 Rice Akitakomachi 5 kg 5kg J4075 Thai Jasmine Rice 25 kg 25kg YTK019 Koshi Yutaka Premium Rice 5 kg 5kg YTK023G Yutaka Sushi Rice 500g 500g YTK033 Brown rice 10kg 10kg NORI & SEAWEED 17164 Dashi Konbu Dried Kelp (Akaya) 1 kg 1kg CN-11-1 Chuka Wakame Seaweed salad 1kg 1kg K1131 Yamanaka Tokuyo Mehijiki - Dry Hijiki Seaweed 25g 25g K1450B Powdered Seaweed Aonori Premium Grade 100g 100g K1619 Seaweed Ogo Nori 500g K1628 Shredded Seaweed Kizami Nori 50g K1697WR Kaiso Mix 100g WR 100g K1703 Ariake Yaki Bara Nori Seaweed Grade A 100g 100g K2903 Seaweed Tosaka Blue 500g K2904 Seaweed Tosaka Red 500g K2905 Seaweed Tosaka White 500g K3214 Kelp Yama Dashi Konbu (Sokusei) 1kg K3217B Kelp Ma Konbu (Hakodate) 500g K3261A Hijiki Seawood - Me Hijiki 1kg K33021 Seaweed - Yakinori Aya Full Size 125g/50sheets K3305 Sushi nori full sheets 100/230G 230g/100sheets K3306 Sushi nori half sheets 100/125g 125g/100sheets K3313 Kofuku nori Seasoned seaweed Ajitsuke Nori Ohavo 8Pkt 24g K3314 Seasoned seaweed sesame nori chips 8G 30/8g K3340 Seaweed Yakinori Miyabi full -

Annual Report 2017 2017 年報 ANNUAL 2 0 REPORT 1 7 年報
(於香港註冊成立的有限公司) (Incorporated in Hong Kong with limited liability) 股份代號 : 1475 Stock Code: 1475 Opening chaa newpter Annual Report 2017 2017 年報 ANNUAL REPORT 2 ANNUAL 0 1 7 年報 Contents 002 Corporate Information 003 Financial Highlights 004 Chairman’s Statement 008 Management Discussion and Analysis 016 Board of Directors and Senior Management 021 Corporate Governance Report 031 Directors’ Report 045 Independent Auditor’s Report Consolidated Statement of Profit or Loss and 049 Other Comprehensive Income The Group maintained its market leadership 050 Consolidated Statement of Financial Position in the instant noodles market in Hong Kong with a balanced portfolio of 052 Consolidated Statement of Changes in Equity products in different price range. Our 054 Consolidated Statement of Cash Flows flagship product brands,Demae Iccho Notes to the Consolidated (出前一丁) and CUP NOODLES (合 056 Financial Statements 味道), continued to be a household 116 Five-year Financial Summary name for the consumers in Hong Kong enjoying high market share given our strong product research & development and innovative mindset. 002 CORPORATE INFORMATION Board of Directors Headquarter and Principal Place of Business Executive Directors 11–13 Dai Shun Street Mr. Kiyotaka Ando Tai Po Industrial Estate Mr. Shinji Tatsutani Tai Po Mr. Munehiko Ono New Territories Mr. Yoshihide Semimaru Hong Kong Mr. Hijiri Fukuoka Auditor Non-executive Director Deloitte Touche Tohmatsu Mr. Tong Ching Hsi Legal Advisors Independent Non-executive Directors CFN lawyers in association with Broad & Bright Dr. Sumio Matsumoto (appointed on 21 November 2017) Broad & Bright Law Firm Mr. Junichi Honda (appointed on 21 November 2017) Anderson Mori & Tomotsune Professor Lynne Yukie Nakano (appointed on 21 November 2017) Company Secretary Principal Bankers Mizuho Bank, Ltd. -
Beef Haw Fun 11 Chicken Lo Mein 9 Yaki Udon 10 Pad Thai
CHARLOTTE, NC Rip.Rip. Dip.Dip. Repeat.Repeat. TwoTwo MalaysianMalaysian flatbreads,flatbreads, servedserved withwith aa sideside ofof ourour signaturesignature currycurry sauce.sauce. 55 Korean Twice Fried Wings 8.5 Sauced in garlic gochujang and topped with peanuts, sesame and cilantro. Hawkers Wings 7.5 BATTERED or NAKED Choice of sauce: sweet thai chili, hainanese , honey sriracha , spring onion ginger or rub: five-spice, chuan jerk Sichuan Wontons 8 Yi-Yi’s Chicken Dumplings 7 Our Auntie’s classic recipe hand-rolled daily, wok-seared or steamed, served with a sweet soy dipping sauce. Golden Wontons 7.5 Hand-made daily filled with chicken, shrimp and mushrooms, fried to a crisp and served with a sweet chili dipping sauce. Char Siu 8 Pork shoulder, sweet soy, spring onions Crispy Tofu Bites 6 Edamame 4 add chili and garlic 1 Hawker’s Delight 7 Saucy blend of wok-seared tofu, broccoli, carrots, napa (pro tip: the cabbage, not the city), bell peppers, shiitake and straw mushrooms Five-Spice Green Beans 6 Lightly battered, fried to a crisp, tossed in our signature five-spice seasoning Chow Faan 9 Classic fried rice packed with chicken, shrimp, char siu, eggs, onions and soy sauce. Basil Fried Rice 7 An herbal take on a classic fave. Eggs, onions, fresh basil and soy sauce. Po Po Lo’s Curry 10 Our hearty family recipe that has been shared for generations with veggies, rice and your choice of: CHICKEN / BEEF / TOFU Jasmine Rice 2 Beef Haw Fun 11 Hey, wok hei! Savory wide rice noodles, sliced steak, green onions, bean sprouts, onions and soy sauce Hokkien Mee 11 Egg noodles mixed with shrimp, sliced chicken and char siu, wok-seared with veggies in a dark soy sauce. -

Instant Noodles As Quotidian and Ubiquitous
IntroDuction Instant Noodles as Quotidian and Ubiquitous Instant ramen noodles—tasty, convenient, cheap, shelf stable, and indus- trially produced (unlike “real” ramen noodles)—are consumed by huge numbers of people worldwide. Invented by Momofuku Ando in 1958, apparently to assist his war-torn Japanese compatriots, they have become so pervasive and commonplace that our friends often expressed surprise at our interest in them. “Why study them?” one asked. “My kids grew up on them.” Were they, in other words, significant enough to be of any particular interest, to be informative about anything of importance? The Noodle Narratives, written by three anthropologists—Deborah and Fred from the United States and Tatsuro from Japan—is our answer to this question and a demonstration that instant noodles are perhaps one of the most remarkable foods ever. The fact that instant noodles are so quotidian and ubiquitous is note- worthy. Their mass-produced ingredients are inexpensive as well as widely available and, for most of the world’s population, broadly acceptable: wheat, vegetable oil, and assorted flavorings, usually includ- ing salt, monosodium glutamate (MSG), a meat or chicken essence, sugar, and flavorings readily blended for local preferences. The World Instant Noodles Association (WINA), created to improve the quality of instant noodles and increase their consumption worldwide, estimates that 95.39 billion packages and cups of instant noodles were sold dur- ing 2010 across an impressive range of markets.1 Almost everyone eats or has eaten instant noodles. 1 Errington - 9780520276338.indd 1 21/05/13 9:33 PM 2 | Introduction Instant noodles are so inexpensive and widespread that they can be used as economic indices. -

Anr 1203 01.Pdf
Annual Report 2012 2012 Report Annual NISSIN FOODS HOLDINGS Annual Report 2012 INVESTOR INFORMATION (NISSIN FOODS HOLDINGS CO., LTD.) As of March 31, 2012 (U.S.$1=¥82.19) Date of Establishment DISTRIBUTION OF OWNERSHIP AMONG SHAREHOLDERS September 1948 Number of Employees 423 (parent company) L Financial Institutions 24.80% WE ARE 7,533 (consolidated basis) L Other Corporations 41.79% Common Stock L Foreign Corporations 14.89% L Individuals and Other 12.30% Authorized: 500,000,000 shares Treasury Stock 6.22% PROUD OF Issued: 117,463,685 shares L Number of Shareholders: 44,188 (Excluding owners of odd-lot shares) PRINCIPAL SHAREHOLDERS OUR PRODUCTS Paid-in Capital ¥25,123 million ($306 million) Number of Percentage of Shares Held Total Shares Name (Thousands) Outstanding Stock Listings Ando Foundation 7,904 6.72% Tokyo Stock Exchange and Mitsubishi Corp. 7,800 6.64 Osaka Securities Exchange ITOCHU Corp. 7,800 6.64 (Ticker Code: 2897) State Street Bank and Trust Company 7,201 6.13 Independent Auditors Ando International Y.K. 4,000 3.40 Mizuho Corporate Bank, Ltd. 3,375 2.87 Deloitte Touche Tohmatsu LLC Japan Trustee Services Bank, Ltd. (Account in Trust) 2,775 2.36 Transfer Agent The Bank of Tokyo-Mitsubishi UFJ, Ltd. 2,629 2.23 Mizuho Trust & Banking Co., Ltd. ONO PHARMACEUTICAL CO., LTD. 2,460 2.09 2-1, Yaesu 1-chome, Chuo-ku, Tokyo, Japan EZAKI GLICO CO., LTD. 2,361 2.00 Total 48,305 41.12 Note: In addition to the above, the Company holds 7,291,193 shares of treasury stock. -

Book Review: the Untold History of Ramen: How Political Crisis in Japan Spawned a Global Food Craze by George Solt
History in the Making Volume 8 Article 17 January 2015 Book Review: The Untold History of Ramen: How Political Crisis in Japan Spawned a Global Food Craze by George Solt. Berkley: University of California Press, 2014. Daniel Stolp CSUSB Follow this and additional works at: https://scholarworks.lib.csusb.edu/history-in-the-making Part of the Asian History Commons Recommended Citation Stolp, Daniel (2015) "Book Review: The Untold History of Ramen: How Political Crisis in Japan Spawned a Global Food Craze by George Solt. Berkley: University of California Press, 2014.," History in the Making: Vol. 8 , Article 17. Available at: https://scholarworks.lib.csusb.edu/history-in-the-making/vol8/iss1/17 This Review is brought to you for free and open access by the History at CSUSB ScholarWorks. It has been accepted for inclusion in History in the Making by an authorized editor of CSUSB ScholarWorks. For more information, please contact [email protected]. Reviews Reviews Book Review: The Untold History of Ramen: How Political Crisis in Japan Spawned a Global Food Craze by George Solt.Fi Berkley: University of California Press, 2014. “Ramen cart in Ueno, Tokyo.” Photograph by Danny Chong Kim Nam. Cover Design by Lia Tjandra. 308 Reviews In The Untold History of Ramen, George Solt explores how something as simple as ramen noodles can be transformed from humble beginnings as a Chinese dish to what is now recognized as a symbol of Japanese culture. Solt shows how the introduction of one simple ingredient can have broad implications not only when it comes to dietary preferences, but in politics and culture as well. -

Nori Tofu Kikkoman Spam Musubi Pocky Dashi Manju Sushi Ramen
Nori Tofu Kikkoman Spam Musubi Pocky Dashi Manju Sushi Ramen Tempura Yakitori Musubi Takoyaki Taiyaki Umeboshi Sashimi Ochazuke Yakisoba Donburi Chirashi Unagi Uni Mochi Tsukemono Anpan Sapporo Ichiban Sake Mikan Bento Gohan Sekihan Dango Miso Teriyaki Kokuho Rose Katsu Curry Ramune Green Tea Kaki / Persimmon Cherry Blossom / Ume / Plum Ozoni Sakura Benihana Daiso Nijiya Marukai Kinokuniya Uniqlo Zojirushi Toyota Nissan Seiko Butsudan Incense / Senko Kodomo no Hi Matsuri Kimono Children's Day Hinamatsuri Koinobori Festival Mochitsuki Oshogatsu Obon Sumo Karaoke Odori Ikebana Taiko Bonsai Mikoshi Shamisen Chanoyu Kenjinkai Fujinkai Ohaka Mairi Executive Order Japan Pearl Harbor 9066 February 19, 1942 December 7, 1941 Assembly Center Day of Topaz Tanforan Remembrance Military Intelligence Manzanar Tule Lake Service (MIS) Civil Liberties Act Dorthea Lange Concentration Camp 442nd Regimental Go For Broke No-No Boy Combat Team Shib Sibs Jake Shimabukuro Purple Heart (Alex & Maia Shibutani) Yuri Kochiyama Eric Shinseki Pat Morita Sessue Hayakawa Ellison Onizuka George Takei Isamu Noguchi Yoko Ono Nikkei Wat(aru) Misaka Norm Mineta Daniel Inouye Tommy Kono Kristi Yamaguchi Apolo Ohno Wally Yonamine Ruth Asawa Fred Korematsu Issei Nisei Sansei Yonsei Hapa Nihonjin Obaachan Ojiichan Sensei (Baachan) (Jiichan) Blanket Boy Angel Island Immigration Gentlemen's Picture Brides Immigration Act Agreement (1965 - abolish national quota) Kodomo no Gambatte Gaman Tame ni Itadakimasu Mottainai Konnichiwa Arigatou Oishii (O)hashi Daruma Tsuru (Crane) Kokeshi Manga Anime Nintendo Playstation Kendama Jan Ken Po Japantown Community Nihonmachi Emperor Empress Samurai Yukata Momotaro Kabuto Geta Tabi Obi.