Mechanisms of Venom Injection and Behavioral Modulation of a Cockroach Prey by a Parasitoid Wasp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Mechanisms Underlying Cockroach Host Manipulation by a Parasitoid Wasp
Molecular Mechanisms Underlying Cockroach Host Manipulation by a Parasitoid Wasp Thesis submitted in partial fulfillment of the requirements for the degree of “DOCTOR OF PHILOSOPHY” by Maayan Kaiser Paltin Submitted to the Senate of Ben-Gurion University of the Negev June 2018 Beer-Sheva Molecular Mechanisms Underlying Cockroach Host Manipulation by a Parasitoid Wasp Thesis submitted in partial fulfillment of the requirements for the degree of “DOCTOR OF PHILOSOPHY” by Maayan Kaiser Paltin Submitted to the Senate of Ben-Gurion University of the Negev Approved by the advisor Approved by the Dean of the Kreitman School of Advanced Graduate Studies June 2018 Beer-Sheva This work was carried out under the supervision of Prof. Frederic Libersat In the Department of Life Sciences Faculty of Natural Sciences Research-Student's Affidavit when Submitting the Doctoral Thesis for Judgment I, Maayan Kaiser Paltin, whose signature appears below, hereby declare that: ___ I have written this Thesis by myself, except for the help and guidance offered by my Thesis Advisors. ___ The scientific materials included in this Thesis are products of my own research, culled from the period during which I was a research student. ___ This Thesis incorporates research materials produced in cooperation with others, excluding the technical help commonly received during experimental work. Therefore, I am attaching another affidavit stating the contributions made by myself and the other participants in this research, which has been approved by them and submitted with their approval. Date: _________________31/12/18 Student's name: ________________Maayan Kaiser Paltin Signature:______________ Affidavit stating the contributions to present work: The venom proteomics which is described at chapter 2, was performed in collaboration with Prof. -

106Th Annual Meeting of the German Zoological Society Abstracts
September 13–16, 2013 106th Annual Meeting of the German Zoological Society Ludwig-Maximilians-Universität München Geschwister-Scholl-Platz 1, 80539 Munich, Germany Abstracts ISBN 978-3-00-043583-6 1 munich Information Content Local Organizers: Abstracts Prof. Dr. Benedikt Grothe, LMU Munich Satellite Symposium I – Neuroethology .......................................... 4 Prof. Dr. Oliver Behrend, MCN-LMU Munich Satellite Symposium II – Perspectives in Animal Physiology .... 33 Satellite Symposium III – 3D EM .......................................................... 59 Conference Office Behavioral Biology ................................................................................... 83 event lab. GmbH Dufourstraße 15 Developmental Biology ......................................................................... 135 D-04107 Leipzig Ecology ......................................................................................................... 148 Germany Evolutionary Biology ............................................................................... 174 www.eventlab.org Morphology................................................................................................ 223 Neurobiology ............................................................................................. 272 Physiology ................................................................................................... 376 ISBN 978-3-00-043583-6 Zoological Systematics ........................................................................... 416 -

Halloween Horrors: the Dark Side of Mother Nature by Francie Mcgowan
Halloween Horrors: The Dark Side of Mother Nature by Francie McGowan As Halloween approaches, monsters, bats and bugs will loom in the darkness of a moonless night in October to scare us. Mother Nature also has some macabre critters of the bug and insect variety that are every bit as eerie and unsettling as any Halloween costume or horror film. [Spoiler alert: do no read this article while eating or you may get sick.] To start with a seemingly pious bug, the praying mantis (order Mantodea), is actually a deadly eater and mater. With her long forelegs she captures her prey and eats them alive while holding them in a death grip. While she is mating, she bites the head of the male clean off and continues chomping the rest of the poor victim until he dies. She then saunters off well fed and fertile. The Japanese giant hornet (Vespa mandarinia japonica) is so big that, in flight, it resembles a small bird. It stings or sprays its victims - including humans - with a flesh-dissolving acid. It usually aims for the eyes. Embedded in this acid is a pheromone that attracts the other hornets in the hive to the victim and they attack en masse. Thirty of these hornets can attack a honey bee hive and kill thirty thousand of them in a matter of a few hours. Another creature to throw the most stalwart person into a state of arachnophobia (fear of spiders) or entomophobia (fear of insects) is the cockroach wasp (Ampulex compressa). They live in Asia and in Africa. -

Children: Especially at Risk with Pests
MAY/JUNE 2014 We accept Pest Mastercard & Visa Patrol CALL TOLL FREE 1-888-744-8989 News Children: Especially at Risk with Pests hile pests affect everyone's health Children are also exposed to fewer germs than Wif they are not controlled, some of especially at risk to pest- adults, their reactions are often us are especially vulnerable to pest stings, induced allergies. Studies more severe. The disease pest transmitted diseases, and pest- have shown that in some organisms pests carry cause caused allergies. This includes our inner city areas, cock- everything from Lyme disease, children, as well as the elderly, and our roaches are the #1 cause of encephalitis and hantavirus, to pets. This article will focus on our children. allergies among children. common diarrhea. Children and infants are especially at An even bigger problem Children, because of their risk to pests because they are always is that children are more small size, are also more at risk moving around and exploring, often close susceptible to the many diseases pests from stinging insects—everything from to the ground or on the ground, causing carry. Dr. Jerome Goddard, a medical bees, hornets, and yellowjackets, to fire them to naturally have more encounters entomologist and author of The ants and bites from poisonous spiders. with pests. Physicians Guide to Arthropods of The same amount of venom from these Rats bite about 45,000 people Medical Importance, points out that insects in a small child can cause a much annually in this country. The vast children are more vulnerable to diseases more serious reaction than in an adult. -

Multifaceted Defense Against Antagonistic Microbes in Developing Offspring of the Parasitoid Wasp Ampulex Compressa (Hymenoptera, Ampulicidae)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Regensburg Publication Server Multifaceted Defense against Antagonistic Microbes in Developing Offspring of the Parasitoid Wasp Ampulex compressa (Hymenoptera, Ampulicidae) Katharina Weiss, Christopher Parzefall, Gudrun Herzner* Evolutionary Ecology Group, Institute of Zoology, University of Regensburg, Regensburg, Germany Abstract Effective antimicrobial strategies are essential adaptations of insects to protect themselves, their offspring, and their foods from microbial pathogens and decomposers. Larvae of the emerald cockroach wasp, Ampulex compressa, sanitize their cockroach hosts, Periplaneta americana, with a cocktail of nine antimicrobials comprising mainly (R)-(-)-mellein and micromolide. The blend of these antimicrobials has broad-spectrum antimicrobial activity. Here we explore the spatio- temporal pattern of deployment of antimicrobials during the development from egg to adult as well as their physico- chemical properties to assess how these aspects may contribute to the success of the antimicrobial strategy. Using gas chromatography/mass spectrometry (GC/MS) we show that larvae start sanitizing their food as soon as they have entered their host to feed on its tissue. Subsequently, they impregnate the cockroach carcass with antimicrobials to create a hygienic substrate for cocoon spinning inside the host. Finally, the antimicrobials are incorporated into the cocoon. The antimicrobial profiles on cockroach and wasp cocoon differed markedly. While micromolide persisted on the cockroaches until emergence of the wasps, solid-phase microextraction sampling and GC/MS analysis revealed that (R)-(-)-mellein vaporized from the cockroaches and accumulated in the enclosed nest. In microbial challenge assays (R)-(-)-mellein in the headspace of parasitized cockroaches inhibited growth of entomopathogenic and opportunistic microbes (Serratia marcescens, Aspergillus sydowii, Metarhizium brunneum). -

Jan 2021 London Zoo Stocklist.Pdf (596.63
ZSL London Zoo - January 2021 stocklist Status at 01.01.2021 m f unk Invertebrata Aurelia aurita * Moon jellyfish 0 0 150 Pachyclavularia violacea * Purple star coral 0 0 1 Tubipora musica * Organ-pipe coral 0 0 2 Pinnigorgia sp. * Sea fan 0 0 20 Sarcophyton sp. * Leathery soft coral 0 0 5 Sinularia sp. * Leathery soft coral 0 0 18 Sinularia dura * Cabbage leather coral 0 0 4 Sinularia polydactyla * Many-fingered leather coral 0 0 3 Xenia sp. * Yellow star coral 0 0 1 Heliopora coerulea * Blue coral 0 0 12 Entacmaea quadricolor Bladdertipped anemone 0 0 1 Epicystis sp. * Speckled anemone 0 0 1 Phymanthus crucifer * Red beaded anemone 0 0 11 Heteractis sp. * Elegant armed anemone 0 0 1 Stichodactyla tapetum Mini carpet anemone 0 0 1 Discosoma sp. * Umbrella false coral 0 0 21 Rhodactis sp. * Mushroom coral 0 0 8 Ricordea sp. * Emerald false coral 0 0 19 Acropora sp. * Staghorn coral 0 0 115 Acropora humilis * Staghorn coral 0 0 1 Acropora yongei * Staghorn coral 0 0 2 Montipora sp. * Montipora coral 0 0 5 Montipora capricornis * Coral 0 0 5 Montipora confusa * Encrusting coral 0 0 22 Montipora danae * Coral 0 0 23 Montipora digitata * Finger coral 0 0 6 Montipora foliosa * Hard coral 0 0 10 Montipora hodgsoni * Coral 0 0 2 Pocillopora sp. * Cauliflower coral 0 0 27 Seriatopora hystrix * Bird nest coral 0 0 8 Stylophora sp. * Cauliflower coral 0 0 1 Stylophora pistillata * Pink cauliflower coral 0 0 23 Catalaphyllia jardinei * Elegance coral 0 0 4 Euphyllia ancora * Crescent coral 0 0 4 Euphyllia glabrescens * Joker's cap coral 0 0 2 Euphyllia paradivisa * Branching frog spawn 0 0 3 Euphyllia paraancora * Branching hammer coral 0 0 3 Euphyllia yaeyamaensis * Crescent coral 0 0 4 Plerogyra sinuosa * Bubble coral 0 0 1 Duncanopsammia axifuga + Coral 0 0 2 Tubastraea sp. -
Jan 2021 ZSL Stocklist.Pdf (699.26
Zoological Society of London - January 2021 stocklist ZSL LONDON ZOO Status at 01.01.2021 m f unk Invertebrata Aurelia aurita * Moon jellyfish 0 0 150 Pachyclavularia violacea * Purple star coral 0 0 1 Tubipora musica * Organ-pipe coral 0 0 2 Pinnigorgia sp. * Sea fan 0 0 20 Sarcophyton sp. * Leathery soft coral 0 0 5 Sinularia sp. * Leathery soft coral 0 0 18 Sinularia dura * Cabbage leather coral 0 0 4 Sinularia polydactyla * Many-fingered leather coral 0 0 3 Xenia sp. * Yellow star coral 0 0 1 Heliopora coerulea * Blue coral 0 0 12 Entacmaea quadricolor Bladdertipped anemone 0 0 1 Epicystis sp. * Speckled anemone 0 0 1 Phymanthus crucifer * Red beaded anemone 0 0 11 Heteractis sp. * Elegant armed anemone 0 0 1 Stichodactyla tapetum Mini carpet anemone 0 0 1 Discosoma sp. * Umbrella false coral 0 0 21 Rhodactis sp. * Mushroom coral 0 0 8 Ricordea sp. * Emerald false coral 0 0 19 Acropora sp. * Staghorn coral 0 0 115 Acropora humilis * Staghorn coral 0 0 1 Acropora yongei * Staghorn coral 0 0 2 Montipora sp. * Montipora coral 0 0 5 Montipora capricornis * Coral 0 0 5 Montipora confusa * Encrusting coral 0 0 22 Montipora danae * Coral 0 0 23 Montipora digitata * Finger coral 0 0 6 Montipora foliosa * Hard coral 0 0 10 Montipora hodgsoni * Coral 0 0 2 Pocillopora sp. * Cauliflower coral 0 0 27 Seriatopora hystrix * Bird nest coral 0 0 8 Stylophora sp. * Cauliflower coral 0 0 1 Stylophora pistillata * Pink cauliflower coral 0 0 23 Catalaphyllia jardinei * Elegance coral 0 0 4 Euphyllia ancora * Crescent coral 0 0 4 Euphyllia glabrescens * Joker's cap coral 0 0 2 Euphyllia paradivisa * Branching frog spawn 0 0 3 Euphyllia paraancora * Branching hammer coral 0 0 3 Euphyllia yaeyamaensis * Crescent coral 0 0 4 Plerogyra sinuosa * Bubble coral 0 0 1 Duncanopsammia axifuga + Coral 0 0 2 Tubastraea sp. -

UNIVERSITY of CALIFORNIA RIVERSIDE Venomics And
UNIVERSITY OF CALIFORNIA RIVERSIDE Venomics and Functional Analysis of Venom From the Emerald Jewel Wasp, Ampulex compressa A Dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Biochemistry and Molecular Biology by Ryan Scott Arvidson June 2016 Dissertation Committee: Dr. Michael E. Adams, Chairperson Dr. Jason Stajich Dr. Anandasankar Ray Copyright by Ryan Scott Arvidson 2016 The Dissertation of Ryan Scott Arvidson is approved: _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ Committee Chairperson University of California, Riverside Acknowledgements Given the adage that “Science does not happen in a vacuum”, I would like to first acknowledge those that contributed to the data presented in this dissertation and who are contributing authors in manuscripts in which this data is to be published. Victor Landa spent considerable time photographic and filming A. compressa in the lab which led to beautiful pictures, as is used in the introductory chapter, and an informative video that is available on the lab’s website (ampulex.ucr.edu). Victor also organized and obtained head capsule size data, and dissected mandibles from A. compressa larva and prepared them for electron microscopy. I would also like to thank Victor for is maintenance of the wasp colony. Sarah Frankenberg dissected and imaged cockroaches containing pupated wasps demonstrating that A. compressa larva is selective in which organs it consumes before pupating. Maayan Kaiser, who at the time of writing this dissertation was a Ph.D. student in the laboratory of Fredric Libersat, at the Ben- Gurion University, Beersheba, Israel, contributed her venom proteomics data in collaboration to generate the A. -
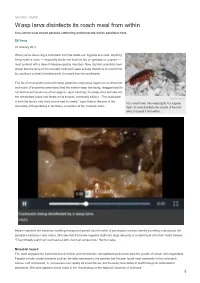
Wasp Larva Disinfects Its Roach Meal from Within Live-Action View Shows Parasite Slathering Antibacterials Inside Paralised Host
NATURE | NEWS Wasp larva disinfects its roach meal from within Live-action view shows parasite slathering antibacterials inside paralised host. Ed Yong 07 January 2013 When you're devouring a cockroach from the inside out, hygiene is a must. Anything living inside a roach — especially inside one that has fed on garbage or corpses — must contend with a slew of disease-causing microbes. Now, German scientists have shown that the larva of the emerald cockroach wasp actively disinfects its roach host by exuding a cocktail of antibacterial chemicals from its mouthparts. The life of an emerald cockroach wasp (Ampulex compressa) begins on an American cockroach (Periplaneta americana) that the mother wasp has stung, dragged back to her burrow and stuck one of her eggs to. Upon hatching, the wasp larva burrows into the immobilized roach and feasts on its innards, eventually killing it. “The cockroach Gudrun Herzner is both the larva’s only food source and its cradle,” says Gudrun Herzner of the For a roach lover, this wasp (right) is a hygiene University of Regensburg in Germany, a member of the research team. freak: its larva disinfects the innards of the host while it devours it from within. Herzner watched the larvae by installing transparent panels into the sides of parasitized roaches, literally providing a window on the parasite’s behaviour (see video). She saw that the larvae regularly slathered large amounts of a clear liquid onto their hosts' tissues. “They virtually soak their cockroaches with chemical compounds,” Herzner says. Microbial hazard The team analysed the liquid and found mellein and micromolide, two substances that can slow the growth of certain microorganisms. -
Hymenoptera, Crabronidae) and the Evolution of an Antimicrobial Brood Protection Mechanism Katharina Weiss1, Erhard Strohm1, Martin Kaltenpoth2,3 and Gudrun Herzner1*
Weiss et al. BMC Evolutionary Biology (2015) 15:291 DOI 10.1186/s12862-015-0565-0 RESEARCH ARTICLE Open Access Comparative morphology of the postpharyngeal gland in the Philanthinae (Hymenoptera, Crabronidae) and the evolution of an antimicrobial brood protection mechanism Katharina Weiss1, Erhard Strohm1, Martin Kaltenpoth2,3 and Gudrun Herzner1* Abstract Background: Hymenoptera that mass-provision their offspring have evolved elaborate antimicrobial strategies to ward off fungal infestation of the highly nutritive larval food. Females of the Afro-European Philanthus triangulum and the South American Trachypus elongatus (Crabronidae, Philanthinae) embalm their prey, paralyzed bees, with a secretion from a complex postpharyngeal gland (PPG). This coating consists of mainly unsaturated hydrocarbons and reduces water accumulation on the prey’s surface, thus rendering it unfavorable for fungal growth. Here we (1) investigated whether a North American Philanthus species also employs prey embalming and (2) assessed the occurrence and morphology of a PPG among females of the subfamily Philanthinae in order to elucidate the evolution of prey embalming as an antimicrobial strategy. Results: We provide clear evidence that females of the North American Philanthus gibbosus possess large PPGs and embalm their prey. The comparative analyses of 26 species from six genera of the Philanthinae, using histological methods and 3D-reconstructions, revealed pronounced differences in gland morphology within the subfamily. A formal statistical analysis based on defined characters of the glands confirmed that while all members of the derived tribe Philanthini have large and complex PPGs, species of the two more basal tribes, Cercerini and Aphilanthopsini, possess simple and comparatively small glands. According to an ancestral state reconstruction, the complex PPG most likely evolved in the last common ancestor of the Philanthini, thus representing an autapomorphy of this tribe. -

March 2020 Issue No
March 2020 Tarsus Issue No. 609 CIRCULAR OF THE ENTOMOLOGICAL SOCIETY OF NEW SOUTH WALES Inc In this edition of Tarsus we give you a teaser for an important up-coming article in General and Applied Entomology. Dr George Bornemissza was an eminent entomologist during the mid to late 20th century, instrumental in the development of the dung beetle program in Australia. George passed away in 2014 but left a large legacy which his wife Jo and close friend Michael Bouffard still continue to bring to fruition. After his retirement to Tasmania George collected a vast array of Coleoptera and embarked on a program to visualise not only the beetles of Tasmania, but the beetles of the world. His aim was to display his insects in both a scientific and artistic way. His early work is on display at both the Tasmanian Museum and Art Gallery in Hobart and the Australian National Insect Collection in Canberra. This month we provide the various financial and other reports for the society: 1. President’s Report 2. Minutes of the 66th Annual General Meeting on 13 March 2019 3. Honorary Secretary’s Report for 2019 4. Statement of Income and Expenditure for the year ended 31 DECEMBER 2019 5. Honorary Treasurer’s Report 6. Council and Committee Members Elected at the Annual General Meeting of 11 March 2020 www.entsocnsw.org.au TARSUS No. 608 January 2020 Page 1 This months member spotlight is Dr George Hangay, who like George Bornemissza, is of Hungarian descent. The two Georges were friends and spent time collecting together and George Hangay has contributed specimens towards the completion of the great work that Jo Bornemissza and Mike Bouffard are undertaking. -

Nitric Oxide Radicals Are Emitted by Wasp Eggs to Kill Mold Fungi Erhard Strohm1*, Gudrun Herzner1, Joachim Ruther2, Martin Kaltenpoth1,3†, Tobias Engl1,3†
RESEARCH ARTICLE Nitric oxide radicals are emitted by wasp eggs to kill mold fungi Erhard Strohm1*, Gudrun Herzner1, Joachim Ruther2, Martin Kaltenpoth1,3†, Tobias Engl1,3† 1Evolutionary Ecology Group, Institute of Zoology, University of Regensburg, Regensburg, Germany; 2Chemical Ecology Group, Institute of Zoology, University of Regensburg, Regensburg, Germany; 3Insect Symbiosis Research Group, Max Planck Institute for Chemical Ecology, Jena, Germany Abstract Detrimental microbes caused the evolution of a great diversity of antimicrobial defenses in plants and animals. Insects developing underground seem particularly threatened. Here we show that the eggs of a solitary digger wasp, the European beewolf Philanthus triangulum, emit large amounts of gaseous nitric oxide (NOÁ) to protect themselves and their provisions, paralyzed honeybees, against mold fungi. We provide evidence that a NO-synthase (NOS) is involved in the generation of the extraordinary concentrations of nitrogen radicals in brood cells (~1500 ppm NOÁ Á and its oxidation product NO2 ). Sequencing of the beewolf NOS gene revealed no conspicuous differences to related species. However, due to alternative splicing, the NOS-mRNA in beewolf eggs lacks an exon near the regulatory domain. This preventive external application of high doses of NOÁ by wasp eggs represents an evolutionary key innovation that adds a remarkable novel facet to the array of functions of the important biological effector NOÁ. DOI: https://doi.org/10.7554/eLife.43718.001 *For correspondence: [email protected]