Original Article Baicalin Down Regulates the Expression of TLR4 and Nfkb-P65 in Colon Tissue in Mice with Colitis Induced by Dextran Sulfate Sodium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Shop Direct Factory List Dec 18
Factory Factory Address Country Sector FTE No. workers % Male % Female ESSENTIAL CLOTHING LTD Akulichala, Sakashhor, Maddha Para, Kaliakor, Gazipur, Bangladesh BANGLADESH Garments 669 55% 45% NANTONG AIKE GARMENTS COMPANY LTD Group 14, Huanchi Village, Jiangan Town, Rugao City, Jaingsu Province, China CHINA Garments 159 22% 78% DEEKAY KNITWEARS LTD SF No. 229, Karaipudhur, Arulpuram, Palladam Road, Tirupur, 641605, Tamil Nadu, India INDIA Garments 129 57% 43% HD4U No. 8, Yijiang Road, Lianhang Economic Development Zone, Haining CHINA Home Textiles 98 45% 55% AIRSPRUNG BEDS LTD Canal Road, Canal Road Industrial Estate, Trowbridge, Wiltshire, BA14 8RQ, United Kingdom UK Furniture 398 83% 17% ASIAN LEATHERS LIMITED Asian House, E. M. Bypass, Kasba, Kolkata, 700017, India INDIA Accessories 978 77% 23% AMAN KNITTINGS LIMITED Nazimnagar, Hemayetpur, Savar, Dhaka, Bangladesh BANGLADESH Garments 1708 60% 30% V K FASHION LTD formerly STYLEWISE LTD Unit 5, 99 Bridge Road, Leicester, LE5 3LD, United Kingdom UK Garments 51 43% 57% AMAN GRAPHIC & DESIGN LTD. Najim Nagar, Hemayetpur, Savar, Dhaka, Bangladesh BANGLADESH Garments 3260 40% 60% WENZHOU SUNRISE INDUSTRIAL CO., LTD. Floor 2, 1 Building Qiangqiang Group, Shanghui Industrial Zone, Louqiao Street, Ouhai, Wenzhou, Zhejiang Province, China CHINA Accessories 716 58% 42% AMAZING EXPORTS CORPORATION - UNIT I Sf No. 105, Valayankadu, P. Vadugapal Ayam Post, Dharapuram Road, Palladam, 541664, India INDIA Garments 490 53% 47% ANDRA JEWELS LTD 7 Clive Avenue, Hastings, East Sussex, TN35 5LD, United Kingdom UK Accessories 68 CAVENDISH UPHOLSTERY LIMITED Mayfield Mill, Briercliffe Road, Chorley Lancashire PR6 0DA, United Kingdom UK Furniture 33 66% 34% FUZHOU BEST ART & CRAFTS CO., LTD No. 3 Building, Lifu Plastic, Nanshanyang Industrial Zone, Baisha Town, Minhou, Fuzhou, China CHINA Homewares 44 41% 59% HUAHONG HOLDING GROUP No. -

Table of Codes for Each Court of Each Level
Table of Codes for Each Court of Each Level Corresponding Type Chinese Court Region Court Name Administrative Name Code Code Area Supreme People’s Court 最高人民法院 最高法 Higher People's Court of 北京市高级人民 Beijing 京 110000 1 Beijing Municipality 法院 Municipality No. 1 Intermediate People's 北京市第一中级 京 01 2 Court of Beijing Municipality 人民法院 Shijingshan Shijingshan District People’s 北京市石景山区 京 0107 110107 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Haidian District of Haidian District People’s 北京市海淀区人 京 0108 110108 Beijing 1 Court of Beijing Municipality 民法院 Municipality Mentougou Mentougou District People’s 北京市门头沟区 京 0109 110109 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Changping Changping District People’s 北京市昌平区人 京 0114 110114 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Yanqing County People’s 延庆县人民法院 京 0229 110229 Yanqing County 1 Court No. 2 Intermediate People's 北京市第二中级 京 02 2 Court of Beijing Municipality 人民法院 Dongcheng Dongcheng District People’s 北京市东城区人 京 0101 110101 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Xicheng District Xicheng District People’s 北京市西城区人 京 0102 110102 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Fengtai District of Fengtai District People’s 北京市丰台区人 京 0106 110106 Beijing 1 Court of Beijing Municipality 民法院 Municipality 1 Fangshan District Fangshan District People’s 北京市房山区人 京 0111 110111 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Daxing District of Daxing District People’s 北京市大兴区人 京 0115 -

List of Designated Supervision Venues for Imported Fruits CNBJS01S008
Firefox https://translate.googleusercontent.com/translate_f List of designated supervision venues for imported fruits Serial Designated supervision site Venue (venue) Off zone mailing address Business unit name number name customs code Beijing Tianzhu Designated Supervision Site 566-5, Shunping Road, Shunyi Comprehensive Bonded 1 Beijing for Inbound Fruits at Capital District, Beijing Zone Development CNBJS01S008 Airport Management Co., Ltd. Inspection site for imported Inspection area in the hospital, Beijing Hutchison Jingtai 2 Beijing fruits at Beijing Chaoyang No.1, East Fourth Ring South Logistics Co., Ltd. CNBJS01S004 Port Road, Beijing Tianjin Port International Tianjin Port International Logistics Designated No. 3016, Yuejin Road, 3 Tianjin Logistics Development Supervision Site for Inbound Tanggu, Binhai New Area CNTXG020444 Co., Ltd. Fruits Tianjin Gangqiang Group's No. 187, Haibin 9th Road, Tianjin Gangqiang Group 4 Tianjin designated supervision site Tianjin Port Free Trade Zone Co., Ltd. CNTXG02S608 for imported fruits Designated Supervision Site Tianjin Dongjiang Port No.601 Handan Road, for Imported Fruits of Large Cold Chain 5 Tianjin Dongjiang Free Trade Port Tianjin Dongjiang Port Commodity Exchange CNDJG02S613 Area, Tianjin Large Cold Chain Market Co., Ltd. No. 1069, Shaanxi Road, Tianjin Dongjiang Shounong Tianjin Port Shounong Tianjin Free Trade Zone 6 Tianjin Food Imported Fruit Food Import and Export (Dongjiang Free Trade Port CNDJG02S614 Designated Supervision Site Trade Co., Ltd. Zone) No. 29, Third Avenue, Airport Designated Supervision Site Sinotrans Cross-border International Logistics Zone, 7 Tianjin for Inbound Fruits at Tianjin E-commerce Logistics Tianjin Pilot Free Trade Zone CNTSN02S609 Binhai Airport Co., Ltd. Tianjin Branch (Airport Economic Zone) Designated Supervision Site Qinhuangdao Sinotrans for Imported Fruits in 71 Youyi Road, Haigang 8 Shijiazhuang International Freight Qinhuangdao Port, Hebei District, Qinhuangdao City CNSHP04S007 Forwarding Co., Ltd. -

Key Projects Push Zhanjiang Forward As Subcenter
12 | Friday, September 18, 2020 CHINA DAILY Transport links to see region rise to prominence By HAO NAN 5.1 Zhanjiang in South China’s Guangdong province is witnessing million major progress in several key con passenger throughout of the struction projects aimed at new airport by 2030 upgrading its transportation infra structure facilities. Another major project is Xuwen In Wuchuan, a countylevel city, Harbor, which is being built to construction on the relocated become a key hub connecting Zhanjiang Airport has moved for Guangdong and Hainan provin ward as planned. ces. The harbor is designed to have The new airport is located 32 an annual handling capacity of 3.2 kilometers away from Zhanjiang’s million vehicles and 17.28 million urban area and 38 km from the passengers. urban area of Maoming, a neigh Construction on the harbor The picturesque Huguangyan Scenic Area in Zhanjiang, Guangdong province. YANG XIAO / FOR CHINA DAILY boring city of Zhanjiang. started on Jan 1, 2017 and is expect In addition to mainly serving ed to be finished for operation in Guangdong’s western areas, the October. When completed, Xuwen airport is also designed for emer Harbor will become a firstclass gency rescue and general aviation and one of the most functional activities. passenger roro ferry harbors in When completed, the new air the world, with seamless connec Key projects push Zhanjiang port will have a runway measuring tion to highways, waterways, rail 3,200 meters and a parallel taxi ways and urban buses. way of equal length, as well as 30 It is of great significance for parking bays, a 61,800squareme serving the construction of Hain ter terminal building and other an Free Trade Port and promoting forward as subcenter supporting facilities. -

(852) 2861-9299
Network Hospital List For assistance or for updated hospital information, you may call the 24-hour IPA Service Hotline. IPA reserves the final right to amend this list of hospitals at any time without prior notice. : (852) 2861-9299 Province/City Hospital Name Address 111 Guangzhou Military Hospital No. 111 Liuhua Road, Guangzhou, Guangdong 48 Guangzhou Huadu District People's No. 48 Xinhua Road, Xinhuazhen, Huadu, Guangzhou Hospital 1838 Southern Hospital No.1838 Dadao North, Guangzhou 253 The First Military Medical University No. 253 Gongyeda Road, Guangzhou Zhujiang Hospital 111 Guangdong Provincial Chinese Medicine No. 111 Dade Road, Guangzhou, Guangdong Hospital 167 Guangzhou Sailor Hospital No. 167 Xingang West Road, Guangzhou, Guangdong 196 Hospital of Guangzhou Economic & No.196 Youyi Road, Guangzhou Economic Technical Development Area Technological Development District Guangdong 1 Guangzhou No.12 People's Hospital No. 1 Xitianqiang Road, Huangpu Road, Guangzhou 65 Guangzhou Panyu Qu Chinese Hospital No. 65 Qiaodong Road, Shiqiao Street, Panyu, Guangzhou Guangdong 19 Eur Am Int l Medical Center No. 19 Huali Road, Zhujiangxincheng, Guangzhou 368 Can Am Medical Center Huayuan Plaza No. 368, Huanshi East Road, Guangzhou Shanwei People's Hospital Lane 2 Min Zhu Plaza, Sanwei, Guangdong Chaoyang People's Hospital Xishuang, Chaoyang, Guangdong 24 Yangchun People's Hospital No. 24 Huangsheng South Road, Chunschengzhen, Yangchun, Guangdong Yangjiang Chinese Medicine Hospital Moyang Street, Jiangcheng District, Yangjiang, Guangdong 2 Yangchun -
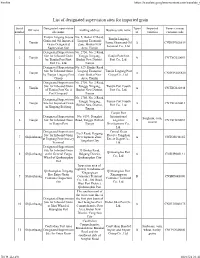
List of Designated Supervision Sites for Imported Grain
Firefox https://translate.googleusercontent.com/translate_f List of designated supervision sites for imported grain Serial Designated supervision Types Imported Venue (venue) Off zone mailing address Business unit name number site name of varieties customs code Tianjin Lingang Jiayue No. 5, Bohai 37 Road, Tianjin Lingang Grain and Oil Imported Lingang Economic 1 Tianjin Jiayue Grain and Oil A CNDGN02S619 Grain Designated Zone, Binhai New Terminal Co., Ltd. Supervision Site Area, Tianjin Designated Supervision No. 2750, No. 2 Road, Site for Inbound Grain Tanggu Xingang, Tianjin Port First 2 Tianjin A CNTXG020051 by Tianjin Port First Binhai New District, Port Co., Ltd. Port Co., Ltd. Tianjin Designated Supervision No. 529, Hunhe Road, Site for Inbound Grain Lingang Economic Tianjin Lingang Port 3 Tianjin A CNDGN02S620 by Tianjin Lingang Port Zone, Binhai New Group Co., Ltd. Group Area, Tianjin Designated Supervision No. 2750, No. 2 Road, Site for Inbound Grain Tanggu Xingang, Tianjin Port Fourth 4 Tianjin A CNTXG020448 of Tianjin Port No. 4 Binhai New District, Port Co., Ltd. Port Company Tianjin No. 2750, No. 2 Road, Designated Supervision Tanggu Xingang, Tianjin Port Fourth 5 Tianjin Site for Imported Grain A CNTXG020413 Binhai New District, Port Co., Ltd. in Xingang Beijiang Tianjin Tianjin Port Designated Supervision No. 6199, Donghai International Sorghum, corn, 6 Tianjin Site for Inbound Grain Road, Tanggu District, Logistics B CNTXG020051 sesame in Tianjin Port Tianjin Development Co., Ltd. Designated Supervision Central Grain No.9 Road, Haigang Site for Inbound Grain Reserve Tangshan 7 Shijiazhuang Development Zone, A CNTGS040165 at Jingtang Port Grocery Direct Depot Co., Tangshan City Terminal Ltd. -
![Directors, Supervisors and Parties Involved in the [Redacted]](https://docslib.b-cdn.net/cover/6866/directors-supervisors-and-parties-involved-in-the-redacted-1826866.webp)
Directors, Supervisors and Parties Involved in the [Redacted]
THIS DOCUMENT IS IN DRAFT FORM, INCOMPLETE AND SUBJECT TO CHANGE AND THAT THE INFORMATION MUST BE READ IN CONJUNCTION WITH THE SECTION HEADED “WARNING” ON THE COVER OF THIS DOCUMENT. DIRECTORS, SUPERVISORS AND PARTIES INVOLVED IN THE [REDACTED] DIRECTORS Name Residential Address Nationality Executive Directors Mr. Wang Jikang ( ) (Chairman) .................. Room 1101, Building J Chinese Central Courtyard Huijing North Street Favorview Palace Tianhe District Guangzhou Guangdong Province PRC Mr. Yi Xuefei ( ) (Vice Chairman) .............. 385-2, Yahuju Agile Garden Chinese 398 Xingnan Avenue Nancun Town Panyu District Guangzhou Guangdong Province PRC Mr. Wu Huiqiang ( ) ........ Room 1604 Chinese No. 38 Taojin Street Yuexiu District Guangzhou Guangdong Province PRC Non-executive Directors Mr. Su Zhigang ( ).......... No.18Zhupo Alley Chinese Gaodi Street Dashi Street Panyu District Guangzhou Guangdong Province PRC Mr. Shao Jianming ( ) ....... No.25Huayuan Yi Street Chinese Yuexiu District Guangzhou Guangdong Province PRC Mr. Li Fangjin ( )........... Room 203, Building 29 Chinese Middle District of South China Normal University No. 55 Zhongshan Road West Tianhe District Guangzhou Guangdong Province PRC 75 THIS DOCUMENT IS IN DRAFT FORM, INCOMPLETE AND SUBJECT TO CHANGE AND THAT THE INFORMATION MUST BE READ IN CONJUNCTION WITH THE SECTION HEADED “WARNING” ON THE COVER OF THIS DOCUMENT. DIRECTORS, SUPERVISORS AND PARTIES INVOLVED IN THE [REDACTED] Name Residential Address Nationality Mr. Zheng Shuping ( )....... Room 2301 Chinese No. 134 Huacheng Road Tianhe District Guangzhou Guangdong Province PRC Mr. Zhu Kelin ( ) ........... Room 2903 Chinese No. 38 Huajing North Street Tianhe District Guangzhou Guangdong Province PRC Mr. Zhang Yongming ( ) ..... 29-3-601 WanQuan Xinxin Jiayuan Chinese Wanliu East Road Haidian District Beijing PRC Mr. -

Shop Direct Factory List Dec 17
FTE No. Factory Name Factory Address Country Sector % M workers (BSQ) BAISHIQING CLOTHING First and Second Area, Donghaian Industrial Zone, Shenhu Town, Jinjiang China CHINA Garments 148 35% (UNITED) ZHUCHENG TIANYAO GARMENTS CO., LTD Zangkejia Road, Textile & Garment Industrial Park, Longdu Subdistrict, Zhucheng City, Shandong Province, China CHINA Garments 332 19% ABHIASMI INTERNATIONAL PVT. LTD Plot No. 186, Sector 25 Part II, Huda, Panipat-132103, Haryana India INDIA Home Textiles 336 94% ABHITEX INTERNATIONAL Pasina Kalan, GT Road Painpat, 132103, Panipat, Haryana, India INDIA Homewares 435 99% ABLE JEWELLERY MFG. LTD Flat A9, West Lianbang Industrial District, Yu Shan xi Road, Panyu, Guangdong Province, China CHINA Jewellery 178 40% ABLE JEWELLERY MFG. LTD Flat A9, West Lianbang Industrial District, Yu Shan xi Road, Panyu, Guangdong Province, China HONG KONG Jewellery 178 40% AFROZE BEDDING UNIT LA-7, Block 22, Federal B Area, Karachi, Pakistan PAKISTAN Home Textiles 980 97% AFROZE TOWEL UNIT Plot No. C-8, Scheme 33, S. I. T.E, Karachi, Sindh, Pakistan PAKISTAN Home Textiles 960 97% AGEME TEKSTIL KONFEKSIYON INS LTD STI (1) Sari Hamazli Mah, 47083 Sok No. 3/2A, Seyhan, Adana, Turkey TURKEY Garments 350 41% AGRA PRODUCTS LTD Plot 94, 99 NSEZ, Phase 2, Noida 201305, U. P., India INDIA Jewellery 377 100% AIRSPRUNG BEDS LTD Canal Road, Canal Road Industrial Estate, Trowbridge, Wiltshire, BA14 8RQ, United Kingdom UK Furniture 398 83% AKH ECO APPARELS LTD 495 Balitha, Shah Belishwer, Dhaamrai, Dhaka, Bangladesh BANGLADESH Garments 5305 56% AL RAHIM Plot A-188, Site Nooriabad, Pakistan PAKISTAN Home Textiles 1350 100% AL-KARAM TEXTILE MILLS PVT LTD Ht-11, Landhi Industrial Area, Karachi. -

A Nomogram to Predict Postoperative Complications in Elderly with Total Hip Replacement
A Nomogram to Predict Postoperative Complications in Elderly with Total Hip Replacement Xiujuan Tan ( [email protected] ) The First Aliated Hospital, Jinan University https://orcid.org/0000-0003-0608-9045 Fengmin Ge The Aliated Hospital of Guangdong Medical University Guixi Mo The Aliated Hospital of Guangdong Medical University Zhiyi Li The Aliated Hospital of Guangdong Medical University Xiaoxia Gu The Aliated Hospital of Guangdong Medical University Liangqing Zhang The Aliated Hospital of Guangdong Medical University Research article Keywords: elderly, total hip replacement, postoperative complication, nomogram Posted Date: October 6th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-79448/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/13 Abstract Background: By analyzing the risk factors of postoperative complications in elderly patients with hip replacement, We aimed to develop a nomogram model based on preoperative and intraoperative variables and veried the sensitivity and specicity for risk stratication of postoperative complications in elderly with total hip replacement patients. Methods: A total of 414 elderly patients who underwent surgical treatment for total hip replacement hospitalized at the Aliated Hospital of Guangdong Medical University from March 1, 2017 to August 31, 2019 were included into this study. Univariate and multivariate logistic regression were conducted to identify independent risk factors of postoperative complication in the 414 patients. A nomogram was developed by R software and validated to predict the risk of postoperative complications. Results: Multivariate logistic regression analysis revealed that age (OR=1.05, 95%CI: 1.00-1.09) , renal failure(OR=0.90, 95% CI: 0.830.97) , Type2 diabetes (OR=1.05, 95% CI: 1.001.09) , ALB (OR=0.91, 95% CI: 0.830.99) were independent risk factors of postoperative complication in elderly patients with hip replacement (P0.05) . -

Key Projects Push Zhanjiang Forward As Subcenter
12 | Friday, September 18, 2020 HONG KONG EDITION | CHINA DAILY Transport links to see region rise to prominence By HAO NAN Zhanjiang in South China’s 5.1 Guangdong province is witnessing million major progress in several key con- passenger throughout of the struction projects aimed at new airport by 2030 upgrading its transportation infra- structure facilities. Another major project is Xuwen In Wuchuan, a county-level city, Harbor, which is being built to construction on the relocated become a key hub connecting Zhanjiang Airport has moved for- Guangdong and Hainan provin- ward as planned. ces. The harbor is designed to have The new airport is located 32 an annual handling capacity of 3.2 kilometers away from Zhanjiang’s million vehicles and 17.28 million urban area and 38 km from the passengers. urban area of Maoming, a neigh- Construction on the harbor The picturesque Huguangyan Scenic Area in Zhanjiang, Guangdong province. YANG XIAO / FOR CHINA DAILY boring city of Zhanjiang. started on Jan 1, 2017 and is expect- In addition to mainly serving ed to be finished for operation in Guangdong’s western areas, the October. When completed, Xuwen airport is also designed for emer- Harbor will become a first-class gency rescue and general aviation and one of the most functional activities. passenger ro-ro ferry harbors in When completed, the new air- the world, with seamless connec- Key projects push Zhanjiang port will have a runway measuring tion to highways, waterways, rail- 3,200 meters and a parallel taxi- ways and urban buses. way of equal length, as well as 30 It is of great significance for parking bays, a 61,800-square-me- serving the construction of Hain- ter terminal building and other an Free Trade Port and promoting forward as subcenter supporting facilities. -

Original Article Knockdown of Lncrna XIST Suppresses Osteosarcoma Progression by Inactivating AKT/Mtor Signaling Pathway by Sponging Mir-375-3P
Int J Clin Exp Pathol 2019;12(5):1507-1517 www.ijcep.com /ISSN:1936-2625/IJCEP0091590 Original Article Knockdown of lncRNA XIST suppresses osteosarcoma progression by inactivating AKT/mTOR signaling pathway by sponging miR-375-3p Xin Sun, Bo Wei, Zhi-Heng Peng, Qing-Long Fu, Chao-Jun Wang, Jin-Chang Zheng, Jie-Cong Sun Orthopaedic Center, Affiliated Hospital of Guangdong Medical University, Xiashan District, Zhanjiang, Guangdong Province, China Received January 21, 2019; Accepted February 22, 2019; Epub May 1, 2019; Published May 15, 2019 Abstract: Background: Osteosarcoma (OS) is one of the most common bone tumors in adolescents and young adults. Emerging evidence suggested ncRNA (lncRNA and miRNA) are closely associated with cell progression, apoptosis and autophagy. However, the role of regulatory network between ncRNA and mRNA in OS has not been fully verified. Methods: lncRNA XIST, miRNA expression were detected by qRT-PCR. The protein expression of LC3, p62, AKT, p-AKT, mTOR and p-mTOR was measured by western blot. MTT assay and flow cytometry were applied to measure cell proliferation and apoptosis. Luciferase assay was used to ensure the relationship between lncRNA, miRNA and mRNA. GFP-LC3 cells were observed using fluorescence microscope. Results: XIST expression was up- regulated but miR-375-3p was down-regulated in OS tissues and cells. Luciferase assay results demonstrated that miR-375-3p was a target of XIST and mTOR was a target mRNA of miR-375-3p. In addition, knockdown of XIST and mTOR inhibited OS cell proliferation and autophagy, but induced apoptosis. Knockdown of XIST could reverse the effect of miR-375-3p inhibitor on OS cells. -

Announcement of Annual Results for the Year Ended 31 December 2019
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this announcement. ANNOUNCEMENT OF ANNUAL RESULTS FOR THE YEAR ENDED 31 DECEMBER 2019 FINANCIAL HIGHLIGHTS • Total contracted sales amounted to a record high of RMB130,030 million, representing an increase of 19% as compared to that of 2018. • Revenue increased by 23% to RMB50,926 million as compared to that of 2018. • Operating profit (excluding fair value gains on investment properties) increased by 14% to RMB10,502 million as compared to that of 2018. • Profit attributable to owners of the Company amounted to RMB2,656 million. Core profit amounted to RMB2,084 million. Basic earnings per share was RMB0.349. • As at 31 December 2019, net gearing ratio was 77% and total cash resources amounted to RMB33,566 million, maintaining financial soundness. • Total assets amounted to RMB243,699 million. • The Board is pleased to propose a final dividend of HKD0.026 per share, in the form of cash. Together with the interim dividend of HKD0.110 per share, total dividend declared for the year was HKD0.136 per share. – 1 – The board of directors (the “Board”) of Sino-Ocean Group Holding Limited (the “Company”) is pleased to announce the audited consolidated results of the Company and its subsidiaries (collectively “our Group”, “the Group” or “we”) for the year ended 31 December 2019.