Wildlife Conservation Act 1950
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Why Is Madagascar Special?
ORIGINAL ARTICLE doi:10.1111/evo.12578 Why is Madagascar special? The extraordinarily slow evolution of pelican spiders (Araneae, Archaeidae) Hannah M. Wood,1,2 Rosemary G. Gillespie,3 Charles E. Griswold,4 and Peter C. Wainwright1 1Department of Evolution and Ecology, University of California, Davis, Davis, California 95616 2E-mail: [email protected] 3Department of Environmental Science, Policy and Management, University of California, Berkeley, Berkeley, California 94720 4Entomology Department, California Academy of Sciences, San Francisco, California 94118 Received May 2, 2014 Accepted November 19, 2014 Although Madagascar is an ancient fragment of Gondwana, the majority of taxa studied thus far appear to have reached the island through dispersal from Cenozoic times. Ancient lineages may have experienced a different history compared to more recent Cenozoic arrivals, as such lineages would have encountered geoclimatic shifts over an extended time period. The motivation for this study was to unravel the signature of diversification in an ancient lineage by comparing an area known for major geoclimatic upheavals (Madagascar) versus other areas where the environment has been relatively stable. Archaeid spiders are an ancient paleoendemic group with unusual predatory behaviors and spectacular trophic morphology that likely have been on Madagascar since its isolation. We examined disparities between Madagascan archaeids and their non-Madagascan relatives regarding timing of divergence, rates of trait evolution, and distribution patterns. Results reveal an increased rate of adaptive trait diversification in Madagascan archaeids. Furthermore, geoclimatic events in Madagascar over long periods of time may have facilitated high species richness due to montane refugia and stability, rainforest refugia, and also ecogeographic shifts, allowing for the accumulation of adaptive traits. -

HEXAPODA INSECTA Australia's Faunal Extinction Crisis Submission
SUPPORTING INFORMATION Table S3 Australian insects and allied invertebrates that have been listed under various conservation schedules, including State/Territory Acts, the EPBC Act and the IUCN Red List, and their occurrence in IBRA regions. Listed species Conservation status Conservation status Conservation status IBRA region (State) (various (EPBC Act 1999) (IUCN Red List 2017) State/Territory Acts) HEXAPODA INSECTA BLATTODEA Panesthia lata Walker, 1868, (Lord Howe Island Endangered PSI (NSW) Wood-feeding Cockroach) (Biodiversity Conservation Act 2016) COLEOPTERA Aulacopris matthewsi Storey, 1986 (Flightless Vulnerable WET (QLD) Dung Beetle) Castiarina insculpta (Carter, 1934) (Miena Jewel Endangered TCH (TAS) Beetle) (Threatened Species Protection Act 1995 Catadromus lacordairei Boisduval , 1835 (Green- Vulnerable FUR, TNM (TAS) lined Ground Beetle) (Threatened Species Protection Act 1995) Enchymus sp. nov. Pascoe, 1871 (Weldborough Rare (Threatened BEL (TAS) Forest Weevil) Species Protection Act 1995) Goedetrechus mendumae Moore, 1972 (Ida Bay Vulnerable TSR (TAS) Cave Beetle) (Threatened Species Protection Act 1995) Goedetrechus parallelus Moore, 1972 (Junee- Vulnerable TWE (TAS) Florentine Cave Beetle) (Threatened Species Protection Act 1995) Hoplogonus bornemisszai Bartolozzi, 1996 Endangered Critically Endangered BEL (TAS) (Bornemissza’s Stag Beetle) (Threatened Species Protection Act 1995 – TAS) Hoplogonus simsoni Parry, 1875 (Simsons Stag Vulnerable Vulnerable BEL, TCH (TAS) Beetle) (Threatened Species Protection Act 1995) Hoplogonus -

Systematic Revision of the Trans-Bassian Moriomorphine Genus
ZooKeys 1044: 339–373 (2021) A peer-reviewed open-access journal doi: 10.3897/zookeys.1044.62335 RESEARCH ARTICLE https://zookeys.pensoft.net Launched to accelerate biodiversity research Systematic revision of the trans-Bassian moriomorphine genus Theprisa Moore (Coleoptera, Carabidae) James K. Liebherr1, Nick Porch2, Matthew Shaw3, Bronte E. Sinclair4, David R. Maddison5 1 Department of Entomology, John H. and Anna B. Comstock Hall, 129 Garden Ave., Cornell University, Ithaca, NY 14853-2601, USA 2 School of Life & Environmental Sciences & Centre for Integrated Ecology, Deakin University, Geelong, VIC 3216, Australia 3 South Australian Museum, South Terrace, Adelaide, SA 5000, Australia 4 Australian National Insect Collection, Building 101, Clunies Ross St., Black Mountain, ACT 2601, Australia 5 Department of Integrative Biology, Oregon State University, Corvallis, OR 97331, USA Corresponding author: James K. Liebherr ([email protected]) Academic editor: Thorsten Assmann | Received 21 December 2020 | Accepted 18 February 2021 | Published 16 June 2021 http://zoobank.org/8264C999-3201-4A3B-B39C-222790AC0192 Citation: Liebherr JK, N Porch, M Shaw, BE Sinclair, DR Maddison (2021) Systematic revision of the trans-Bassian moriomorphine genus Theprisa Moore (Coleoptera, Carabidae). In: Spence J, Casale A, Assmann T, Liebherr JK, Penev L (Eds) Systematic Zoology and Biodiversity Science: A tribute to Terry Erwin (1940–2020). ZooKeys 1044: 339–373. https://doi.org/10.3897/zookeys.1044.62335 Abstract The Australian genus Theprisa Moore, 1963, is taxonomically revised to comprise five species, two newly described: Theprisa darlingtoniLiebherr & Porch, sp. nov. of Tasmania, and Theprisa otway Liebherr, Porch & Maddison, sp. nov. from the Otway Ranges, Victoria. Two previously described species, T. -

Checklist of Australian Spiders (Total of 3,935 Species in 677 Genera and 87 Families* by Volker W
Checklist of Australian Spiders (total of 3,935 species in 677 genera and 87 families* by Volker W. Framenau Version 1.43: Saturday, 17 October 2020 For feedback and corrections please contact: [email protected] *The family Stenochilidae occurs in Australia (Framenau, Baehr, Zborowski 2014) but since no species have been published for this country, this family is currently not listed with any species below. this page: Araneomorphae Agelenidae Oramia Araneomorphae Agelenidae C. L. Koch, 1837 Modern Funnel-web Spiders Oramia Forster, 1964 frequens (Rainbow, 1920) Tegenaria Latreille, 1804 domestica (Clerck, 1758) Amaurobiidae Thorell, 1870 Mesh-web Weavers Dardurus Davies, 1976 agrestis Davies, 1976 nemoralis Davies, 1976 saltuosus Davies, 1976 silvaticus Davies, 1976 spinipes Davies, 1976 tamborinensis Davies, 1976 Daviesa Koçal & Kemal, 2008 gallonae (Davies, 1993) lubinae (Davies, 1993) Oztira Milledge, 2011 affinis (Hickman, 1981) aquilonaria (Davies, 1986) kroombiti Milledge, 2011 summa (Davies, 1986) Storenosoma Hogg, 1900 altum Davies, 1986 bifidum Milledge, 2011 bondi Milledge, 2011 forsteri Milledge, 2011 grayi Milledge, 2011 grossum Milledge, 2011 hoggi (Roewer, 1942) picadilly Milledge, 2011 smithae Milledge, 2011 supernum Davies, 1986 tasmaniensis Milledge, 2011 terraneum Davies, 1986 Page 1 of 100 this page: Araneomorphae Amaurobiidae Storenosoma victoria Milledge, 2011 Tasmabrochus Davies, 2002 cranstoni Davies, 2002 montanus Davies, 2002 turnerae Davies, 2002 Tasmarubrius Davies, 1998 hickmani Davies, 1998 milvinus -

Uncovering a Secret World on the South Coast
Page header?? UNCOVERING A SECRET WORLD ON THE SOUTH COAST Hiding beneath leaf litter and under rocks on the south coast lies a hidden world of spineless animals. Thanks to the tireless work of the South Coast Threatened Invertebrate Group we are getting a better picture of these amazing animals and how best to protect them. by Sarah Comer, Melinda Moir, Mark Harvey and Deon Utber LANDSCOPE 47 he south-west of Western published scientific literature each year Previous page Australia is recognised as one of 34 due to the many species requiring names Main Mark Harvey collecting invertebrates global biodiversity hotspots, and and the few taxonomists available to do at Middle Island off the south coast. T Photo – Sarah Comer/Parks and Wildlife while the flora and vertebrate fauna are the job. The term ‘Linnaean Shortfall’ Bottom The WA pill millipede (Cynotelopus relatively well described, the challenge of describes this bottleneck situation, but notabilis). documenting invertebrate diversity is one doesn’t help to identify which species that has kept the South Coast Threatened might require conservation. This is partly Above A female Zephyrarchaea mainae found Invertebrate Group (SCTIG) members achieved by conducting surveys and at Torndirrup National Park. Photos – Michael Rix/WA Museum busy for nearly 15 years. examining records to determine how restricted species actually are, where UNDERSTANDING THE they occur and, just as importantly, SPINELESS MAJORITY where they do not occur. This lack of To fully comprehend the challenges of knowledge on a species biogeography is Australia and Parks and Wildlife sought to invertebrate conservation it is important referred to as the ‘Wallacean Shortfall’. -
Published Version
PUBLISHED VERSION Michael G. Rix, Mark S. Harvey Australian assassins, Part II: a review of the new assassin spider genus Zephyrarchaea (Araneae, Archaeidae) from southern Australia ZooKeys, 2012; 191(SPL.ISS.):1-62 © 2012 Michael G. Rix. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Originally published at: http://doi.org/10.3897/zookeys.191.3070 PERMISSIONS CC BY 3.0 http://creativecommons.org/licenses/by/3.0/ http://hdl.handle.net/2440/86523 A peer-reviewed open-access journal ZooKeys 191:Australian 1–62 (2012) Assassins, Part II: A review of the new assassin spider genus Zephyrarchaea... 1 doi: 10.3897/zookeys.191.3070 MONOGRAPH www.zookeys.org Launched to accelerate biodiversity research Australian Assassins, Part II: A review of the new assassin spider genus Zephyrarchaea (Araneae, Archaeidae) from southern Australia Michael G. Rix1,†, Mark S. Harvey1,2,3,4,‡ 1 Department of Terrestrial Zoology, Western Australian Museum, Locked Bag 49, Welshpool DC, Perth, We- stern Australia 6986, Australia 2 Research Associate, Division of Invertebrate Zoology, American Museum of Natural History, New York, NY 10024, USA 3 Research Associate, California Academy of Sciences, 55 Music Concourse Drive, San Francisco, CA 94118, USA 4 Adjunct Professor, School of Animal Biology, University of Western Australia, 35 Stirling Highway, Crawley, Perth, Western Australia 6009, Australia † urn:lsid:zoobank.org:author:B7D4764D-B9C9-4496-A2DE-C4D16561C3B3 ‡ urn:lsid:zoobank.org:author:FF5EBAF3-86E8-4B99-BE2E-A61E44AAEC2C Corresponding author: Michael G. -

Wood MPE 2018.Pdf
Molecular Phylogenetics and Evolution 127 (2018) 907–918 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Next-generation museum genomics: Phylogenetic relationships among palpimanoid spiders using sequence capture techniques (Araneae: T Palpimanoidea) ⁎ Hannah M. Wooda, , Vanessa L. Gonzáleza, Michael Lloyda, Jonathan Coddingtona, Nikolaj Scharffb a Smithsonian Institution, National Museum of Natural History, 10th and Constitution Ave. NW, Washington, D.C. 20560-0105, U.S.A. b Biodiversity Section, Center for Macroecology, Evolution and Climate, Natural History Museum of Denmark, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen, Denmark ARTICLE INFO ABSTRACT Keywords: Historical museum specimens are invaluable for morphological and taxonomic research, but typically the DNA is Ultra conserved elements degraded making traditional sequencing techniques difficult to impossible for many specimens. Recent advances Exon in Next-Generation Sequencing, specifically target capture, makes use of short fragment sizes typical of degraded Ethanol DNA, opening up the possibilities for gathering genomic data from museum specimens. This study uses museum Araneomorphae specimens and recent target capture sequencing techniques to sequence both Ultra-Conserved Elements (UCE) and exonic regions for lineages that span the modern spiders, Araneomorphae, with a focus on Palpimanoidea. While many previous studies have used target capture techniques on dried museum specimens (for example, skins, pinned insects), this study includes specimens that were collected over the last two decades and stored in 70% ethanol at room temperature. Our findings support the utility of target capture methods for examining deep relationships within Araneomorphae: sequences from both UCE and exonic loci were important for resolving relationships; a monophyletic Palpimanoidea was recovered in many analyses and there was strong support for family and generic-level palpimanoid relationships. -
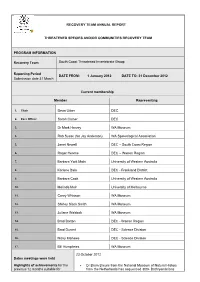
Recovery Team Annual Report Threatened Species
RECOVERY TEAM ANNUAL REPORT THREATENED SPECIES AND/OR COMMUNITIES RECOVERY TEAM PROGRAM INFORMATION Recovery Team South Coast Threatened Invertebrate Group Reporting Period DATE FROM: 1 January 2012 DATE TO: 31 December 2012 Submission date 31 March Current membership Member Representing 1. Chair Deon Utber DEC 2. Exec Officer Sarah Comer DEC 3. Dr Mark Harvey WA Museum 4. Rob Susac (for Jay Anderson) WA Speleological Association 5. Janet Newell DEC – South Coast Region 6. Roger Hearne DEC – Warren Region 7. Barbara York Main University of Western Australia 8. Karlene Bain DEC - Frankland District 9. Barbara Cook University of Western Australia 10. Melinda Moir University of Melbourne 11. Corey Whisson WA Museum 12. Shirley Slack Smith WA Museum 13. Juliane Waldock WA Museum 14. Brad Barton DEC - Warren Region 15. Brad Durant DEC - Science Division 16. Nicky Marlowe DEC - Science Division 17. Bill Humphries WA Museum 23 October 2012 Dates meetings were held Highlights of achievements for the Dr Bram Breure from the National Museum of Natural History previous 12 months suitable for from the Netherlands has sequenced 300+ Bothryembrions publication in WATSNU and specimens for DNA including specimens recently collected from contribution to DEC annual report. the South Coast. Provide 1-2 paragraphs summarising Zephyrarchaea nominations - Mark Harvey and Mike Rix from total number of new populations the WA Museum have reviewed the nominations and four of the located, surveys completed, list described species fit criteria for threatened under IUCN – Z. major management actions etc barretaee, Z. marki, Z. melindae, Z. robinsi. The other two WA species are Z. mainae (to remain as threatened) and a western species – Z. -

The Extraordinarily Slow Evolution of Pelican Spiders (Araneae, Archaeidae)
UC Berkeley UC Berkeley Previously Published Works Title Why is Madagascar special? The extraordinarily slow evolution of pelican spiders (Araneae, Archaeidae). Permalink https://escholarship.org/uc/item/90k031g5 Journal Evolution; international journal of organic evolution, 69(2) ISSN 0014-3820 Authors Wood, Hannah M Gillespie, Rosemary G Griswold, Charles E et al. Publication Date 2015-02-01 DOI 10.1111/evo.12578 Peer reviewed eScholarship.org Powered by the California Digital Library University of California ORIGINAL ARTICLE doi:10.1111/evo.12578 Why is Madagascar special? The extraordinarily slow evolution of pelican spiders (Araneae, Archaeidae) Hannah M. Wood,1,2 Rosemary G. Gillespie,3 Charles E. Griswold,4 and Peter C. Wainwright1 1Department of Evolution and Ecology, University of California, Davis, Davis, California 95616 2E-mail: [email protected] 3Department of Environmental Science, Policy and Management, University of California, Berkeley, Berkeley, California 94720 4Entomology Department, California Academy of Sciences, San Francisco, California 94118 Received May 2, 2014 Accepted November 19, 2014 Although Madagascar is an ancient fragment of Gondwana, the majority of taxa studied thus far appear to have reached the island through dispersal from Cenozoic times. Ancient lineages may have experienced a different history compared to more recent Cenozoic arrivals, as such lineages would have encountered geoclimatic shifts over an extended time period. The motivation for this study was to unravel the signature of diversification in an ancient lineage by comparing an area known for major geoclimatic upheavals (Madagascar) versus other areas where the environment has been relatively stable. Archaeid spiders are an ancient paleoendemic group with unusual predatory behaviors and spectacular trophic morphology that likely have been on Madagascar since its isolation. -

Royal Society of Western Australia, 97: 57–64, 2014
WA Science—Journal of the Royal Society of Western Australia, 97: 57–64, 2014 Arachnida (Arthropoda: Chelicerata) of Western Australia: overview and prospects M S HARVEY * Department of Terrestrial Zoology, Western Australian Museum, Locked Bag 49, Welshpool DC, WA 6986, Australia. [email protected]. The history of the study of arachnids (spiders, scorpions, ticks, mites and their relatives) in Western Australia is briefly reviewed, and the main periods of activity are documented: 1860s–1910s, between the wars, after World War II, and the modern era. The fauna consists of at least 1400 named species (but the mite fauna is imperfectly documented), and it is estimated that ~6000 species exist, the majority of which are currently undescribed KEYWORDS: history, pseudoscorpions, scorpions, spiders, taxonomy. INTRODUCTION nowadays known as Missulena granulosum (Cambridge). This species is quite common throughout southwestern The arachnid fauna of Western Australia represents a Australia where it persists in woodland habitats. The fascinating tableau of ancient relictual species and more next arachnid to be described was Idiops blackwalli recently arrived invaders. While the spiders (order Cambridge (1870) based on an adult male collected from Araneae) and mites (superorder Acari) are numerically Swan River. The species was quickly transferred to a new dominant, representatives of six other orders— genus by Ausserer (1871). Idiommata blackwalli is a large, Scorpiones, Pseudoscorpiones, Opiliones, Schizomida, impressive species still common in the Perth region. The Amblypygi and Palpigradi (Figure 1)—have been found trapdoor spiders Aganippe latior (based on a female from in the state. The size of the fauna is unknown, but ‘West Australia’), Eriodon insignis (based on a male from certainly comprises several thousand species, the Swan River), and E. -

Checklist of Australian Spiders (Total of 3,839 Species in 674 Genera and 82 Families*) by Volker W
Checklist of Australian Spiders (total of 3,839 species in 674 genera and 82 families*) by Volker W. Framenau Version 1.41: Friday, 27 July 2018 For feedback and corrections please contact: [email protected] Acknowledgements (thanks for feedback to): Gustavo Hormiga (Tetragnathidae), Rob Whyte (several typographic errors and species omissions), Mark Harvey (Oecobiidae), Jürgen Otto (Salticidae), Barbara Baehr (Orsolobidae). *The family Stenochilidae occurs in Australia (Framenau, Baehr, Zborowski 2014) but since no species have been published for this country, this family is currently not listed with any species below. this page: Araneomorphae Agelenidae Oramia Araneomorphae Agelenidae C. L. Koch, 1837 Modern Funnel-web Spiders Oramia Forster, 1964 frequens (Rainbow, 1920) Tegenaria Latreille, 1804 domestica (Clerck, 1758) Amaurobiidae Thorell, 1870 Mesh-web Weavers Dardurus Davies, 1976 agrestis Davies, 1976 nemoralis Davies, 1976 saltuosus Davies, 1976 silvaticus Davies, 1976 spinipes Davies, 1976 tamborinensis Davies, 1976 Daviesa Koçal & Kemal, 2008 gallonae (Davies, 1993) lubinae (Davies, 1993) Oztira Milledge, 2011 affinis (Hickman, 1981) aquilonaria (Davies, 1986) kroombiti Milledge, 2011 summa (Davies, 1986) Storenosoma Hogg, 1900 altum Davies, 1986 bifidum Milledge, 2011 bondi Milledge, 2011 forsteri Milledge, 2011 grayi Milledge, 2011 grossum Milledge, 2011 hoggi (Roewer, 1942) picadilly Milledge, 2011 smithae Milledge, 2011 supernum Davies, 1986 tasmaniensis Milledge, 2011 terraneum Davies, 1986 Page 1 of 98 this page: -

Flora and Fauna Survey Report R001 JBS&G
Planning Solutions Ocean Beach Telstra Phone Tower Lot 555 Ocean Beach Road Ocean Beach WA 10 February 2020 Flora and Fauna Survey Report R001 JBS&G Table of Contents 1. Introduction ................................................................................................................... 1 1.1 Background .......................................................................................................... 1 1.2 Scope ................................................................................................................... 1 2. Context .......................................................................................................................... 3 2.1 Legislative context ............................................................................................... 3 2.1.1 EPBC Act ............................................................................................... 3 2.1.2 BC Act ................................................................................................... 3 2.1.3 EP Act ................................................................................................... 3 2.1.4 BAM Act ............................................................................................... 4 2.2 Other Legislation relevant to the MBS Site ......................................................... 4 2.2.1 Telecommunications Act ...................................................................... 4 3. Environmental setting ..................................................................................................