Protuss Liquid.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

General Pharmacology
GENERAL PHARMACOLOGY Winners of “Nobel” prize for their contribution to pharmacology Year Name Contribution 1923 Frederick Banting Discovery of insulin John McLeod 1939 Gerhard Domagk Discovery of antibacterial effects of prontosil 1945 Sir Alexander Fleming Discovery of penicillin & its purification Ernst Boris Chain Sir Howard Walter Florey 1952 Selman Abraham Waksman Discovery of streptomycin 1982 Sir John R.Vane Discovery of prostaglandins 1999 Alfred G.Gilman Discovery of G proteins & their role in signal transduction in cells Martin Rodbell 1999 Arvid Carlson Discovery that dopamine is neurotransmitter in the brain whose depletion leads to symptoms of Parkinson’s disease Drug nomenclature: i. Chemical name ii. Non-proprietary name iii. Proprietary (Brand) name Source of drugs: Natural – plant /animal derivatives Synthetic/semisynthetic Plant Part Drug obtained Pilocarpus microphyllus Leaflets Pilocarpine Atropa belladonna Atropine Datura stramonium Physostigma venenosum dried, ripe seed Physostigmine Ephedra vulgaris Ephedrine Digitalis lanata Digoxin Strychnos toxifera Curare group of drugs Chondrodendron tomentosum Cannabis indica (Marijuana) Various parts are used ∆9Tetrahydrocannabinol (THC) Bhang - the dried leaves Ganja - the dried female inflorescence Charas- is the dried resinous extract from the flowering tops & leaves Papaver somniferum, P album Poppy seed pod/ Capsule Natural opiates such as morphine, codeine, thebaine Cinchona bark Quinine Vinca rosea periwinkle plant Vinca alkaloids Podophyllum peltatum the mayapple -

Jp Xvii the Japanese Pharmacopoeia
JP XVII THE JAPANESE PHARMACOPOEIA SEVENTEENTH EDITION Official from April 1, 2016 English Version THE MINISTRY OF HEALTH, LABOUR AND WELFARE Notice: This English Version of the Japanese Pharmacopoeia is published for the convenience of users unfamiliar with the Japanese language. When and if any discrepancy arises between the Japanese original and its English translation, the former is authentic. The Ministry of Health, Labour and Welfare Ministerial Notification No. 64 Pursuant to Paragraph 1, Article 41 of the Law on Securing Quality, Efficacy and Safety of Products including Pharmaceuticals and Medical Devices (Law No. 145, 1960), the Japanese Pharmacopoeia (Ministerial Notification No. 65, 2011), which has been established as follows*, shall be applied on April 1, 2016. However, in the case of drugs which are listed in the Pharmacopoeia (hereinafter referred to as ``previ- ous Pharmacopoeia'') [limited to those listed in the Japanese Pharmacopoeia whose standards are changed in accordance with this notification (hereinafter referred to as ``new Pharmacopoeia'')] and have been approved as of April 1, 2016 as prescribed under Paragraph 1, Article 14 of the same law [including drugs the Minister of Health, Labour and Welfare specifies (the Ministry of Health and Welfare Ministerial Notification No. 104, 1994) as of March 31, 2016 as those exempted from marketing approval pursuant to Paragraph 1, Article 14 of the Same Law (hereinafter referred to as ``drugs exempted from approval'')], the Name and Standards established in the previous Pharmacopoeia (limited to part of the Name and Standards for the drugs concerned) may be accepted to conform to the Name and Standards established in the new Pharmacopoeia before and on September 30, 2017. -

Medication Guide for a Safe Recovery
Medication Guide For A Safe Recovery A guide to maintaining sobriety while receiving treatment for other health problems. Revision 1.0 -April 2008 Table of Contents Introduction..................................................................................2 How to Use this Guide..................................................................3 Class A Drugs (Absolutely Avoid)................................................4 Class B Drugs................................................................................8 (With Addiction Medicine Specialist/Doctor Approval Only) Class C Drugs (Generally Safe to Take).....................................12 Alcohol-Free Products..................................................................16 Incidental Exposure Index...........................................................22 www.talbottcampus.com Introduction From the Talbott Recovery Campus Welcome to the Talbott Recovery Campus guide for a safe and sustained recovery. This document was developed through a collaborative effort between some of the best minds in addiction care today and will help you make wise decisions, ensuring that medications you may be prescribed and incidental exposure to alcohol do not threaten your hard won recovery. This guide is divided into three sections and is based on the drug classification system developed nearly 20 years ago by Dr. Paul Earley and recently expanded on by Bruce Merkin, M.D., Renee Enstrom, Nicholas Link and the staff at Glenbeigh hospital. Part one provides a way of categorizing medications -
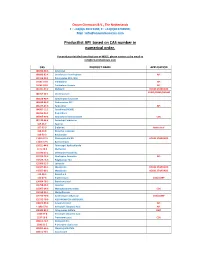
Productlist API Based on CAS Number in Numerical Order
Onium Chemicals B.V., The Netherlands T.: +31(0)6 20171394; F.: +31(0)847233093; Mail : [email protected] Productlist API based on CAS number in numerical order. For products detailed specifications or MSDS, please contact us by email to [email protected] CAS PRODUCT NAME APPLICATION 100286-90-6 Irinotecan 100986-85-4 Levofloxacin hemihydrate API 101418-00-2 Policresulen 35%, 50% 10161-33-8 Trenbolone API 10161-34-9 Trenbolone Acetate API 101831-37-2 Diclazuril HOUSE STANDARD CGMP/EDMF/USDMF 102767-28-2 Levetiracetam 103628-48-4 Sumatriptan Succinate 103639-04-9 Ondansetron HCl 104227-87-4 Famciclovir API 104987-11-3 Tacrolimus(FK506) 106266-06-2 Risperidone 107007-99-8 Granisetron hydrochloride COS 107133-36-8 Perindopril erbumine 107-35-7 Taurine 107-95-9 β-alanine Amino acid 108-59-8 Dimethyl malonate 109-73-9 Butylamine 11015-37-5 Flavomycin 4% 8% HOUSE STANDARD 11015-37-5 Bambermycin 110221-44-8 Temocapril hydrochloride 1115-70-4 Metformin 111696-23-2 Cefetamet Pivoxil HCL 111974-72-2 Quetiapine Fumarate API 112529-15-4 Pioglitazone HCl 112809-51-5 Letrozole 113507-06-5 Moxidectin HOUSE STANDARD 113507-06-5 Moxidectin HOUSE STANDARD 113-98-4 Penicillin G 114-07-8 Erythromycin COS/CGMP 114084-78-5 Ibandronic acid 114798-26-4 Losartan 115007-34-6 Mycophenolate mofetil COS 115550-35-1 Marbofloxacin 117772-70-0 Azithromycin dihydrate COS/CGMP 117772-70-0 AZITHROMYCIN DIHYDRATE 118072-93-8 Zoledronic Acid API 11808-37-0 N-Acetyl-L-Glutamic Acid API 118443-89-3 Cefquinome Sulfate DMF 1188-37-0 N-Acetyl-L-Glutamic Acid 1197-18-8 -

Alphabetical Listing of ATC Drugs & Codes
Alphabetical Listing of ATC drugs & codes. Introduction This file is an alphabetical listing of ATC codes as supplied to us in November 1999. It is supplied free as a service to those who care about good medicine use by mSupply support. To get an overview of the ATC system, use the “ATC categories.pdf” document also alvailable from www.msupply.org.nz Thanks to the WHO collaborating centre for Drug Statistics & Methodology, Norway, for supplying the raw data. I have intentionally supplied these files as PDFs so that they are not quite so easily manipulated and redistributed. I am told there is no copyright on the files, but it still seems polite to ask before using other people’s work, so please contact <[email protected]> for permission before asking us for text files. mSupply support also distributes mSupply software for inventory control, which has an inbuilt system for reporting on medicine usage using the ATC system You can download a full working version from www.msupply.org.nz Craig Drown, mSupply Support <[email protected]> April 2000 A (2-benzhydryloxyethyl)diethyl-methylammonium iodide A03AB16 0.3 g O 2-(4-chlorphenoxy)-ethanol D01AE06 4-dimethylaminophenol V03AB27 Abciximab B01AC13 25 mg P Absorbable gelatin sponge B02BC01 Acadesine C01EB13 Acamprosate V03AA03 2 g O Acarbose A10BF01 0.3 g O Acebutolol C07AB04 0.4 g O,P Acebutolol and thiazides C07BB04 Aceclidine S01EB08 Aceclidine, combinations S01EB58 Aceclofenac M01AB16 0.2 g O Acefylline piperazine R03DA09 Acemetacin M01AB11 Acenocoumarol B01AA07 5 mg O Acepromazine N05AA04 -

(12) Patent Application Publication (10) Pub. No.: US 2002/0102215 A1 100 Ol
US 2002O102215A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2002/0102215 A1 Klaveness et al. (43) Pub. Date: Aug. 1, 2002 (54) DIAGNOSTIC/THERAPEUTICAGENTS (60) Provisional application No. 60/049.264, filed on Jun. 6, 1997. Provisional application No. 60/049,265, filed (75) Inventors: Jo Klaveness, Oslo (NO); Pal on Jun. 6, 1997. Provisional application No. 60/049, Rongved, Oslo (NO); Anders Hogset, 268, filed on Jun. 7, 1997. Oslo (NO); Helge Tolleshaug, Oslo (NO); Anne Naevestad, Oslo (NO); (30) Foreign Application Priority Data Halldis Hellebust, Oslo (NO); Lars Hoff, Oslo (NO); Alan Cuthbertson, Oct. 28, 1996 (GB)......................................... 9622.366.4 Oslo (NO); Dagfinn Lovhaug, Oslo Oct. 28, 1996 (GB). ... 96223672 (NO); Magne Solbakken, Oslo (NO) Oct. 28, 1996 (GB). 9622368.0 Jan. 15, 1997 (GB). ... 97OO699.3 Correspondence Address: Apr. 24, 1997 (GB). ... 9708265.5 BACON & THOMAS, PLLC Jun. 6, 1997 (GB). ... 9711842.6 4th Floor Jun. 6, 1997 (GB)......................................... 97.11846.7 625 Slaters Lane Alexandria, VA 22314-1176 (US) Publication Classification (73) Assignee: NYCOMED IMAGING AS (51) Int. Cl." .......................... A61K 49/00; A61K 48/00 (52) U.S. Cl. ............................................. 424/9.52; 514/44 (21) Appl. No.: 09/765,614 (22) Filed: Jan. 22, 2001 (57) ABSTRACT Related U.S. Application Data Targetable diagnostic and/or therapeutically active agents, (63) Continuation of application No. 08/960,054, filed on e.g. ultrasound contrast agents, having reporters comprising Oct. 29, 1997, now patented, which is a continuation gas-filled microbubbles stabilized by monolayers of film in-part of application No. 08/958,993, filed on Oct. -

Pharmacy Data Management Drug Exception List
Pharmacy Data Management Drug Exception List Patch PSS*1*127 updated the following drugs with the listed NCPDP Multiplier and NCPDP Dispense Unit. These two fields were added as part of this patch to the DRUG file (#50). Please refer to the Release notes for ePharmacy/ECME Enhancements for Pharmacy Release Notes (BPS_1_5_EPHARMACY_RN_0907.PDF) on the VistA Documentation Library (VDL). The IEN column reflects the IEN for the VA PRODUCT file (#50.68). The ePharmacy Change Control Board provided the following list of drugs with the specified NCPDP Multiplier and NCPDP Dispense Unit values. This listing was used to update the DRUG file (#50) with a post install routine in the PSS*1*127 patch. NCPDP File 50.68 NCPDP Dispense IEN Product Name Multiplier Unit 2 ATROPINE SO4 0.4MG/ML INJ 1.00 ML 3 ATROPINE SO4 1% OINT,OPH 3.50 GM 6 ATROPINE SO4 1% SOLN,OPH 1.00 ML 7 ATROPINE SO4 0.5% OINT,OPH 3.50 GM 8 ATROPINE SO4 0.5% SOLN,OPH 1.00 ML 9 ATROPINE SO4 3% SOLN,OPH 1.00 ML 10 ATROPINE SO4 2% SOLN,OPH 1.00 ML 11 ATROPINE SO4 0.1MG/ML INJ 1.00 ML 12 ATROPINE SO4 0.05MG/ML INJ 1.00 ML 13 ATROPINE SO4 0.4MG/0.5ML INJ 1.00 ML 14 ATROPINE SO4 0.5MG/ML INJ 1.00 ML 15 ATROPINE SO4 1MG/ML INJ 1.00 ML 16 ATROPINE SO4 2MG/ML INJ 1.00 ML 18 ATROPINE SO4 2MG/0.7ML INJ 0.70 ML 21 ATROPINE SO4 0.3MG/ML INJ 1.00 ML 22 ATROPINE SO4 0.8MG/ML INJ 1.00 ML 23 ATROPINE SO4 0.1MG/ML INJ,SYRINGE,5ML 5.00 ML 24 ATROPINE SO4 0.1MG/ML INJ,SYRINGE,10ML 10.00 ML 25 ATROPINE SO4 1MG/ML INJ,AMP,1ML 1.00 ML 26 ATROPINE SO4 0.2MG/0.5ML INJ,AMP,0.5ML 0.50 ML 30 CODEINE PO4 30MG/ML -

BCBSM Clinical Drug List (Formulary)
Blue Cross Blue Shield of Michigan ® ® Clinical Drug List (Formulary) Please note that this listing of medications contained in this Blue Cross Blue Shield of Michigan Clinical Drug List (Formulary) is current at the time that the list is posted to this website, and is subject to change. INTRODUCTION Blue Cross Blue Shield of Michigan is pleased to provide the Clinical Drug List as a useful reference and educational tool to assist providers in selecting cost-effective therapies. Please familiarize yourself with this information. To provide effective high-quality care, this Clinical Drug List requires the continuing support of physicians and phar - macists. Your questions and suggestions are welcome. PREFACE The Blue Cross Blue Shield of Michigan Clinical Drug List is a list of FDA-approved prescription drug medications reviewed by the BCBSM/BCN Pharmacy and Therapeutics (P&T) Committee. The Clinical Drug List will assist in maintaining the quality of patient care and containing cost for the member’s drug benefit plan. Providers, physicians, and pharmacists are encouraged to refer to the Clinical Drug List when selecting prescription drug therapy for eligible plan members. Physicians are encouraged to prescribe med ications included in the Clinical Drug List whenever possible. If a prescrip tion is written for a nonpreferred drug or for a drug or dose not recommended for use in the elderly or pregnant, phar macists are encouraged to contact the physician. The benefit plan administrator will monitor provider- specific drug list prescribing and communicate with providers to optimize compliance. The Clinical Drug List is divided into major therapeutic categories (chapters) for easy use. -

(12) Patent Application Publication (10) Pub. No.: US 2007/0141147 A1 Heil Et Al
US 200701.41 147A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2007/0141147 A1 Heil et al. (43) Pub. Date: Jun. 21, 2007 (54) SEQUENTIAL RELEASE Publication Classification PHARMACEUTICAL FORMULATIONS (51) Int. Cl. (75) Inventors: Matthew F. Heil, Duluth, GA A6II 3/55 (2006.01) (US); Glynn Wilson, Duluth, GA A63L/485 (2006.01) (US s s A6IR 9/22 (2006.01) A63/37 (2006.01) Correspondence Address: AOIN 31/08 (2006.01) PATREAL PABST (52) U.S. Cl. ........ 424/468; 514/282: 514/304: 514/649; PABST PATENT GROUP LLP 514/217.05: 514/731 400 COLONY SQUARE, SUITE 1200, 1201 s PEACHTREE STREET ATLANTA, GA 30361 (57) ABSTRACT A mixed-release tablet or capsule formulation including (73) Assignee: Auriga Laboratories, Inc. vehicles for the delivery of a plurality of drugs in various combinations of immediate release, extended release, and/or (21) Appl. No.: 11/461,238 delayed release modes over a predetermined time period 1-1. have been developed, which provide for controlled release (22) Filed: Jul. 31, 2006 not just of the drugs, but controlled release that is designed O O to create more effective coordination between the drugs Related U.S. Application Data being delivered. The drugs can be any medically and/or (60) Provisional application No. 60/752,057, filed on Dec. physiologically appropriate combination of drugs and active 21, 2005, provisional application No. 60/761,766, ingredients, preferably decongestant drugs, antihistamines, filed on Jan. 25, 2006, provisional application No. expectorants, antitussives, cough Suppressants, and drying 60/791,408, filed on Apr.