Piperazine Derivatives As Dangerous Abused Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Substance Abuse: the Pharmacy Educator’S Role in Prevention and Recovery
Substance Abuse: The Pharmacy Educator’s Role in Prevention and Recovery Curricular Guidelines for Pharmacy: Substance Abuse and Addictive Disease i Curricular Guidelines for Pharmacy: Substance Abuse and Addictive Disease1,2 BACKGROUND OF THE CURRICULUM DEVELOPMENT PROJECT In 1988, the AACP Special Interest Group (SIG) on Pharmacy Student and Faculty Impairment (renamed Substance Abuse Education and Assistance) undertook the development of curricular guidelines for colleges/schools of pharmacy to facilitate the growth of educational opportunities for student pharmacists. These Curricular Guidelines for Pharmacy Education: Substance Abuse and Addictive Disease were published in 1991 (AJPE. 55:311-16. Winter 1991.) One of the charges of the Special Committee on Substance Abuse and Pharmacy Education was to review and revise the 1991 curricular guidelines. Overall, the didactic and experiential components in the suggested curriculum should prepare the student pharmacist to competently problem-solve issues concerning alcohol and other drug abuse and addictive diseases affecting patients, families, colleagues, themselves, and society. The guidelines provide ten educational goals, while describing four major content areas including: psychosocial aspects of alcohol and other drug use; pharmacology and toxicology of abused substances; identification, intervention, and treatment of people with addictive diseases; and legal/ethical issues. The required curriculum suggested by these guidelines addresses the 1 These guidelines were revised by the AACP Special Committee on Substance Abuse and Pharmacy Education. Members drafting the revised guidelines were Edward M. DeSimone (Creighton University), Julie C. Kissack (Harding University), David M. Scott (North Dakota State University), and Brandon J. Patterson (University of Iowa). Other Committee members were Paul W. Jungnickel, Chair (Auburn University), Lisa A. -

(19) United States (12) Patent Application Publication (10) Pub
US 20130289061A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0289061 A1 Bhide et al. (43) Pub. Date: Oct. 31, 2013 (54) METHODS AND COMPOSITIONS TO Publication Classi?cation PREVENT ADDICTION (51) Int. Cl. (71) Applicant: The General Hospital Corporation, A61K 31/485 (2006-01) Boston’ MA (Us) A61K 31/4458 (2006.01) (52) U.S. Cl. (72) Inventors: Pradeep G. Bhide; Peabody, MA (US); CPC """"" " A61K31/485 (201301); ‘4161223011? Jmm‘“ Zhu’ Ansm’ MA. (Us); USPC ......... .. 514/282; 514/317; 514/654; 514/618; Thomas J. Spencer; Carhsle; MA (US); 514/279 Joseph Biederman; Brookline; MA (Us) (57) ABSTRACT Disclosed herein is a method of reducing or preventing the development of aversion to a CNS stimulant in a subject (21) App1_ NO_; 13/924,815 comprising; administering a therapeutic amount of the neu rological stimulant and administering an antagonist of the kappa opioid receptor; to thereby reduce or prevent the devel - . opment of aversion to the CNS stimulant in the subject. Also (22) Flled' Jun‘ 24’ 2013 disclosed is a method of reducing or preventing the develop ment of addiction to a CNS stimulant in a subj ect; comprising; _ _ administering the CNS stimulant and administering a mu Related U‘s‘ Apphcatlon Data opioid receptor antagonist to thereby reduce or prevent the (63) Continuation of application NO 13/389,959, ?led on development of addiction to the CNS stimulant in the subject. Apt 27’ 2012’ ?led as application NO_ PCT/US2010/ Also disclosed are pharmaceutical compositions comprising 045486 on Aug' 13 2010' a central nervous system stimulant and an opioid receptor ’ antagonist. -

Programme & Abstracts
The 57th Annual Meeting of the International Association of Forensic Toxicologists. 2nd - 6th September 2019 BIRMINGHAM, UK The ICC Birmingham Broad Street, Birmingham B1 2EA Programme & Abstracts 1 Thank You to our Sponsors PlatinUm Gold Silver Bronze 2 3 Contents Welcome message 5 Committees 6 General information 7 iCC maps 8 exhibitors list 10 Exhibition Hall 11 Social Programme 14 opening Ceremony 15 Schedule 16 Oral Programme MONDAY 2 September 19 TUESDAY 3 September 21 THURSDAY 5 September 28 FRIDAY 6 September 35 vendor Seminars 42 Posters 46 oral abstracts 82 Poster abstracts 178 4 Welcome Message It is our great pleasure to welcome you to TIAFT Gala Dinner at the ICC on Friday evening. On the accompanying pages you will see a strong the UK for the 57th Annual Meeting of scientific agenda relevant to modern toxicology and we The International Association of Forensic thank all those who submitted an abstract and the Toxicologists Scientific Committees for making the scientific programme (TIAFT) between 2nd and 6th a success. Starting with a large Young Scientists September 2019. Symposium and Dr Yoo Memorial plenary lecture by Prof Tony Moffat on Monday, there are oral session topics in It has been decades since the Annual Meeting has taken Clinical & Post-Mortem Toxicology on Tuesday, place in the country where TIAFT was founded over 50 years Human Behaviour Toxicology & Drug-Facilitated Crime on ago. The meeting is supported by LTG (London Toxicology Thursday and Toxicology in Sport, New Innovations and Group) and the UKIAFT (UK & Ireland Association of Novel Research & Employment/Occupational Toxicology Forensic Toxicologists) and we thank all our exhibitors and on Friday. -

Ecstasy Or Molly, Is a Synthetic Psychoactive Drug
DRUG ENFORCEMENT ADMINISTRATION THE FACTS ABOUT MDMA Ecstasy &Molly DEA PHOTO What is it? MDMA, (3,4-methylenedioxy- methamphetamine), known as Ecstasy or Molly, is a synthetic psychoactive drug. Ecstasy is the pill form of MDMA. Molly is the slang for “molecular” that refers to the powder or crystal form of MDMA. Molly is often mixed with other drugs and substances and is not pure MDMA or safe to use. How is it used? MDMA or Ecstasy is taken orally in pill or tablet form. These pills can be in different colors with images on them. Molly is taken in a gel capsule or snorted. What does MDMA do to the body and mind? • As a stimulant drug, it increases heart rate and blood pressure. Users may experience muscle tension, involuntary teeth clenching, nausea, blurred vision, faintness, chills, or sweating • It produces feelings of increased energy, euphoria, emotional warmth, empathy, and distortions in sensory and time perception. • Feelings of sadness, anxiety, depression and memory difficulties are other effects. • It can seriously deplete serotonin levels in the brain, causing confusion and sleep problems. Did you know? • DEA has labeled MDMA as a Schedule I drug, meaning its abuse potential is high and it has no approved medical use. It is illegal in the U.S. • In high doses, MDMA can affect the body’s ability to regulate temperature, which can lead to serious health complications and possible death. • Teens are using less MDMA. Teens decreased their past year use of MDMA from 1.9% in 2010 to 1.2% of teens using 2012. -

Psychoactive Substances and Transpersonal States
TRANSPERSONAL PSYCHOLOGY RESEARCH REVIEW: PSYCHOACTIVE SUBSTANCES AND TRANSPERSONAL STATES David Lukoff San Francisco, California Robert Zanger Los Angeles, California Francis Lu San Francisco, California This "Research Review" covers recent trends in researching psychoactive substances and trans personal states of conscious ness during the past ten years. In keeping with the stated goals of this section of the Journal to promote research in transpersonal psychology, the focus is on the methods and trends designs which are being employed in investigations rather than during the findings on this topic. However, some recently published the books and monographs provide good summaries of the recent last findings relevant to understanding psychoactive substances ten (Cohen & Krippner, 1985b; Dobkin de Rios, 1984;Dobkin de years Rios & Winkelman, 1989b; Ratsch, 1990; Reidlinger, 1990). Because researching psychoactive substances is most broadly a cross-disciplinary venture, only a small portion of the research reviewed below was conducted by persons who consider The authors wish to acknowledge the assistance of Bruce Flath, head librarian at the California Institute of Integral Studies, San Francisco in conducting the computer bibliographic searches used in preparation of this article. The authors also wish to thank Marlene Dobkin de Rios, Stanley Krippner, Christel Lukoff, Dennis McKenna, Terence McKenna, Ralph Metzner, Donald Rothberg and Ilene Serlin for their valuable comments on earlier drafts of this article. Copyright © 1990 Transpersonal Institute The Journal of Transpersonal Psychology. 1990, Vol. 22, No.2 107 themselves transpersonal psychologists. As Vaughan (1984) has noted, "The transpersonal perspective is a meta-perspec tive, an attempt to learn from all different disciplines . emerging from the needed integration of ancient wisdom and modern science. -

WO 2015/072852 Al 21 May 2015 (21.05.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/072852 Al 21 May 2015 (21.05.2015) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 36/84 (2006.01) A61K 31/5513 (2006.01) kind of national protection available): AE, AG, AL, AM, A61K 31/045 (2006.01) A61P 31/22 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 31/522 (2006.01) A61K 45/06 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, PCT/NL20 14/050780 KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (22) International Filing Date: MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, 13 November 2014 (13.1 1.2014) PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (25) Filing Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 61/903,430 13 November 2013 (13. 11.2013) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: RJG DEVELOPMENTS B.V. -
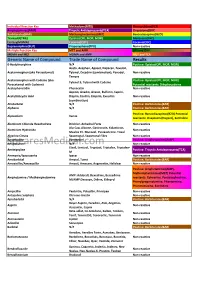
Cross Reaction Guide
Individual Reaction Key Methadone(MTD) Phencyclidine(PCP) Amphetamines(AMP) Tricyclic Antidepressants(TCA) Oxycodone(OXY) Barbiturates(BAR) Methamphetamines(MET) Benzodiazepines(BZO) Tramadol(TML) Opiates(OPI, MOP, MOR) Marijuana(THC) Ecstasy(MDMA) Cotinine(COT) Cocaine(COC) Buprenorphine(BUP) Propoxyphene(PPX) Non-reactive Multiple Reaction Key MET and AMP OPI and OXY MDMA and MET MDMA and AMP MET and TCA Generic Name of Compound Trade Name of Compound Results 6-Acetylmorphine N/A Positive: Opiates(OPI, MOP, MOR) Aceta, Acephen, Apacet, Dapacen, Feverall, Acetaminophen (aka Paracetamol) Tylenol, Excedrin (combination), Panadol, Non-reactive Tempra Acetaminophen with Codeine (aka Positive: Opiates(OPI, MOP, MOR) Tylenol 3, Tylenol with Codeine Paracetamol with Codeine) Potential reactants: Dihydrocodeine Acetophenetidin Phenacetin Non-reactive Aspirin, Anadin, Anasin, Bufferin, Caprin, Acetylsalicyclic Acid Disprin, Ecotrin, Empirin, Excedrin Non-reactive (combination) Allobarbital N/A Positive: Barbiturates(BAR) Alphenol N/A Positive: Barbiturates(BAR) Positive: Benzodiazepines(BZO) Potential Alprazolam Xanax reactants: Oxaprozin (Daypro), Sertraline Aluminum Chloride Hexahydrate Drichlor, Anhydrol Forte Non-reactive Alu-Cap, Alisone, Gastrocote, Kolanticon, Aluminum Hydroxide Non-reactive Maalox TC, Mucogel, Pyrogastrone, Topal Alverine Citrate Spasmonal, Spasmonal Fibre Non-reactive Amantadine Symmetrel Positive: Amphetamines(AMP) SpearesMedical.comAminopyrine N/A Non-reactive Elavil, Lentizol, Tryptizol, Triptafen, Triptafen- Amitriptyline -

10 Facts About MDMA
10 Facts About MDMA August 2015 1. What is MDMA? and a desire to intensify these feelings by dancing, talking and touching. MDMA, often referred to as “ecstasy” or “molly”, is short for 3,4 methylenedioxymethamphetamine, a A typical dose of 80 - 125 mg lasts three to six hours. psychoactive drug derived from safrole oil. MDMA Some people experience nausea at the outset, but produces effects that resemble both stimulants and after about 45 minutes, report feelings of relaxation psychedelics, as well as its signature effect: a feeling and clarity. MDMA also causes dilation of the pupils of connectedness. It impacts brain function primarily and, often, sensitivity to light, as well as possible jaw- releasing the neurotransmitter serotonin, and also clenching, tooth-grinding, muscle tension, faintness, temporarily inhibits its reuptake. MDMA is usually and chills or sweating. taken orally, whether in pressed pill form, powder or crystal; or sometimes snorted. After the drug wears off, the theory from preclinical studies is that brain levels of serotonin (a chemical MDMA was originally synthesized in 1912 by the drug responsible for maintaining mood balance) are company Merck.1 However, its psychoactive effects depleted, which can lead in some cases to sadness, weren’t widely discovered until 1976 when Alexander anxiety, depression and sleep problems.4 If they occur, Shulgin developed a new synthesis method, tested the these symptoms arise in the several days that follow. drug on himself, and shared it with a few friendly Generally, they abate within a week, though frequency psychotherapists.2 Because of the drug’s effects of of use and higher doses can slow or stop this increasing empathy and reducing fear, it started to be process.5 used in psychotherapy practices in the 1970s and early 80s, as well as recreationally. -

References to Argentina
References to Argentina Part 1 RECENT STATISTICS AND TREND ANALYSIS OF ILLICIT DRUG MARKETS A. EXTENT OF ILLICIT DRUG USE AND HEALTH CONSEQUENCES The Americas (pages 12 to 14 WDR 13) In the Americas, a high prevalence of most illicit drugs, essentially driven by estimates in North America, was observed, with the prevalence of cannabis (7.9 per cent) and cocaine (1.3 per cent) being particularly high in the region. South America, Central America and the Caribbean The annual prevalence of cocaine use in South America (1.3 per cent of the adult population) is comparable to levels in North America, while it remains much higher than the global average in Central America (0.6 per cent) and the Caribbean (0.7 per cent). Cocaine use has increased significantly in Brazil, Costa Rica and, to lesser extent, Peru while no change in its use was reported in Argentina. The use of cannabis in South America is higher (5.7 per cent) than the global average, but lower in Central America and Caribbean (2.6 and 2.8 per cent respectively). In South America and Central America the use of opioids (0.3 and 0.2 per cent, respectively) and Ecstasy (0.1 per cent each) also remain well below the global average. While opiates use remains low, countries such as Colombia report that heroin use is becoming increasingly common among certain age groups and socio-economic classes.30 Part 2 NEW PSYCHOACTIVE SUBSTANCES C. THE RECENT EMERGENCE AND SPREAD OF NEW PSYCHOACTIVE SUBSTANCES (page 67 WDR 13) Spread at the global level Number of countries reporting the emergence of new psychoactive substances Pursuant to Commission on Narcotic Drugs resolution 55/1, entitled “Promoting international cooperation in responding to the challenges posed by new psychoactive substances”, in 2012 UNODC sent a questionnaire on NPS to all Member States, to which 80 countries and territories replied. -

Piperazine (MDBZP)
1-(3-4- methylendioxybenzyl)piperazine (MDBZP) Pre-Review Report Expert Committee on Drug Dependence Thirty-fifth Meeting Hammamet, Tunisia, 4-8 June 2012 35th ECDD (2012) Agenda item 5.3e 1-(3-4-methylendioxybenzyl)piperazine (MDBZP) - 2 - 35th ECDD (2012) Agenda item 5.3e 1-(3-4-methylendioxybenzyl)piperazine (MDBZP) Acknowledgements This report has been drafted under the responsibility of the WHO Secretariat, Essential Medicines and Health Products, Medicines Access and Rational Use Unit. The WHO Secretariat would like to thank the following people for their contribution in producing this pre-review report: Dr Simon Elliott, United Kingdom (literature review and drafting), Dr Caroline Bodenschatz (editing) and Mr Kamber Celebi, France (questionnaire report). - 3 - 35th ECDD (2012) Agenda item 5.3e 1-(3-4-methylendioxybenzyl)piperazine (MDBZP) - 4 - 35th ECDD (2012) Agenda item 5.3e 1-(3-4-methylendioxybenzyl)piperazine (MDBZP) Contents SUMMARY ............................................................................................................................... 7 1. Substance identification .................................................................................................... 8 A. International Nonproprietary Name (INN) ................................................................ 8 B. Chemical Abstract Service (CAS) Registry Number ................................................. 8 C. Other Names .............................................................................................................. -

HANDBOOK of Medicinal Herbs SECOND EDITION
HANDBOOK OF Medicinal Herbs SECOND EDITION 1284_frame_FM Page 2 Thursday, May 23, 2002 10:53 AM HANDBOOK OF Medicinal Herbs SECOND EDITION James A. Duke with Mary Jo Bogenschutz-Godwin Judi duCellier Peggy-Ann K. Duke CRC PRESS Boca Raton London New York Washington, D.C. Peggy-Ann K. Duke has the copyright to all black and white line and color illustrations. The author would like to express thanks to Nature’s Herbs for the color slides presented in the book. Library of Congress Cataloging-in-Publication Data Duke, James A., 1929- Handbook of medicinal herbs / James A. Duke, with Mary Jo Bogenschutz-Godwin, Judi duCellier, Peggy-Ann K. Duke.-- 2nd ed. p. cm. Previously published: CRC handbook of medicinal herbs. Includes bibliographical references and index. ISBN 0-8493-1284-1 (alk. paper) 1. Medicinal plants. 2. Herbs. 3. Herbals. 4. Traditional medicine. 5. Material medica, Vegetable. I. Duke, James A., 1929- CRC handbook of medicinal herbs. II. Title. [DNLM: 1. Medicine, Herbal. 2. Plants, Medicinal.] QK99.A1 D83 2002 615′.321--dc21 2002017548 This book contains information obtained from authentic and highly regarded sources. Reprinted material is quoted with permission, and sources are indicated. A wide variety of references are listed. Reasonable efforts have been made to publish reliable data and information, but the author and the publisher cannot assume responsibility for the validity of all materials or for the consequences of their use. Neither this book nor any part may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, microfilming, and recording, or by any information storage or retrieval system, without prior permission in writing from the publisher. -

The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances
WHO/PSM/QSM/2006.3 The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances 2006 Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines Medicines Policy and Standards The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 © World Health Organization 2006 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.