Cyanobacteria Commonly Form Blooms on the Surface of Productive, Warm, Stable Lakes and Reservoirs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RURAL ECONOMY Ciecnmiiuationofsiishiaig Activity Uthern All
RURAL ECONOMY ciEcnmiIuationofsIishiaig Activity uthern All W Adamowicz, P. BoxaIl, D. Watson and T PLtcrs I I Project Report 92-01 PROJECT REPORT Departmnt of Rural [conom F It R \ ,r u1tur o A Socio-Economic Evaluation of Sportsfishing Activity in Southern Alberta W. Adamowicz, P. Boxall, D. Watson and T. Peters Project Report 92-01 The authors are Associate Professor, Department of Rural Economy, University of Alberta, Edmonton; Forest Economist, Forestry Canada, Edmonton; Research Associate, Department of Rural Economy, University of Alberta, Edmonton and Research Associate, Department of Rural Economy, University of Alberta, Edmonton. A Socio-Economic Evaluation of Sportsfishing Activity in Southern Alberta Interim Project Report INTROI)UCTION Recreational fishing is one of the most important recreational activities in Alberta. The report on Sports Fishing in Alberta, 1985, states that over 340,000 angling licences were purchased in the province and the total population of anglers exceeded 430,000. Approximately 5.4 million angler days were spent in Alberta and over $130 million was spent on fishing related activities. Clearly, sportsfishing is an important recreational activity and the fishery resource is the source of significant social benefits. A National Angler Survey is conducted every five years. However, the results of this survey are broad and aggregate in nature insofar that they do not address issues about specific sites. It is the purpose of this study to examine in detail the characteristics of anglers, and angling site choices, in the Southern region of Alberta. Fish and Wildlife agencies have collected considerable amounts of bio-physical information on fish habitat, water quality, biology and ecology. -

Vessel Operation Restriction Regulations Règlement Sur Les Restrictions Visant L’Utilisation Des Bâtiments
CANADA CONSOLIDATION CODIFICATION Vessel Operation Restriction Règlement sur les restrictions Regulations visant l’utilisation des bâtiments SOR/2008-120 DORS/2008-120 Current to June 20, 2019 À jour au 20 juin 2019 Last amended on October 10, 2018 Dernière modification le 10 octobre 2018 Published by the Minister of Justice at the following address: Publié par le ministre de la Justice à l’adresse suivante : http://laws-lois.justice.gc.ca http://lois-laws.justice.gc.ca OFFICIAL STATUS CARACTÈRE OFFICIEL OF CONSOLIDATIONS DES CODIFICATIONS Subsections 31(1) and (3) of the Legislation Revision and Les paragraphes 31(1) et (3) de la Loi sur la révision et la Consolidation Act, in force on June 1, 2009, provide as codification des textes législatifs, en vigueur le 1er juin follows: 2009, prévoient ce qui suit : Published consolidation is evidence Codifications comme élément de preuve 31 (1) Every copy of a consolidated statute or consolidated 31 (1) Tout exemplaire d'une loi codifiée ou d'un règlement regulation published by the Minister under this Act in either codifié, publié par le ministre en vertu de la présente loi sur print or electronic form is evidence of that statute or regula- support papier ou sur support électronique, fait foi de cette tion and of its contents and every copy purporting to be pub- loi ou de ce règlement et de son contenu. Tout exemplaire lished by the Minister is deemed to be so published, unless donné comme publié par le ministre est réputé avoir été ainsi the contrary is shown. publié, sauf preuve contraire. -

Wabamun Lake Water Quality 1982 to 2001
WABAMUN LAKE WATER QUALITY 1982 TO 2001 WABAMUN LAKE WATER QUALITY 1982 TO 2001 Prepared by: Richard Casey, M.Sc. Limnologist Science and Standards Alberta Environment September 2003 W0309 Pub. No: T/695 ISBN: 0-7785-2503-1 (Printed Edition) ISBN: 0-7785-2504-X (On-Line Edition) Web Site: http://www3.gov.ab.ca/env/info/infocentre/publist.cfm Any comments, questions, or suggestions regarding the content of this document may be directed to: Environmental Monitoring and Evaluation Branch Alberta Environment 10th Floor, Oxbridge Place 9820 – 106th Street Edmonton, Alberta T5K 2J6 Phone: (780) 427-6278 Fax: (780) 422-6712 Additional copies of this document may be obtained by contacting: Information Centre Alberta Environment Main Floor, Great West Life Building 9920 – 108th Street Edmonton, Alberta T5K 2M4 Phone: (780) 944-0313 Fax: (780) 427-4407 Email: [email protected] SUMMARY Wabamun Lake, approximately 60 km west of Edmonton, is large, shallow, and generally well mixed. Sport fish in the lake include northern pike, yellow perch, and lake whitefish. There are a unique mix of land uses in the lake watershed, which include undisturbed bush and forest, agriculture, two coal mines with active and reclaimed areas, three coal-fired power plants, major transportation (road and rail) corridors, residences, and recreation. The mines supply fuel for the power plants, operated by the TransAlta Utilities Corporation (TAU). Industrial wastewaters, runoff and cooling water from the Whitewood mine and Wabamun power plant are discharged to the lake. Over time, TAU operations associated with the mines and power plants in the watershed have caused cumulative and ongoing impacts on the lake level. -

Eutrophication Processes in Alberta Lakes
Eutrophication processes in Alberta lakes Alexander P. Wolfe Department of Earth & Atmospheric Sciences University of Alberta, Edmonton <[email protected]> Grand Beach Lake Winnipeg Eutrophication processes in Alberta lakes • A general model for prairie lakes • Coupling of multiple elemental cycles • Coupling of inorganic and biological processes • An over-arching context involving climate/hydrological changes • Dramatic consequences for surface water quality EUTROPHICATION : The state of lakes under nutrient enrichment Grand Beach Lake Winnipeg EUTROPHICATION 20 µg/L Very common in Alberta and across the prairies; Typically accompanied by: • algal blooms • high chlorophyll • reduced biodiversity • anoxia • fish kills • esthetics and Alberta SRD property values The faces of eutrophic lakes A key role for phosphorus (P) control Experimental Lakes Area, Ontario, 1970’s, 80’s D.W. Schindler P added P concentrations >20 µg/L engender eutrophication culprits: urban and agricultural runoff, septic failures, golf courses, etc. 2 pH rises ; .) aq O + + O O 2( 2 CH 2 = ↑ O O 2 pH ∆ + + H 2 CO [P] drivesproduction algal [P] depletes Photosynthesis CO • • ves primary production Dri Chemicalconsequences: bio-inorganic bridging What goes around comes around • When algae die and settle on sediments, respiration of organic matter consumes dissolved O2, produces CO2, and pH drops as H2CO3 is produced: CH2O + O2 CO2 + H2O CO2 + H2O H2CO3 Pipit Lake, Alberta Why is this important ? • The delicate balance between oxidizing and reducing conditions (REDOX) ultimately determines the range of chemical reactions possible in lakes • In many Alberta lakes, the cycling of IRON (Fe) and SULFUR (P) can become critical in locking up (sequestering) or releasing (diffusing) PHOSPHORUS (P) stored in sediments. -

Moose Lake Handbook
Moose Lake Our Past - Our Home - Our Future Moose Lake has a rich history starting with the fur trade and continually evolving to today with over 10,000 visitors annually. The area is under pressure from development such as subdivisions, lakelots, campgrounds, industry, agriculture and recreation; as well as the wildlife that make the lake their home. The Moose Lake Watershed Society (MLWS) recognizes that watershed management is vital to conserving the lake and maintaining its ecological value that we all can enjoy. The Moose Lake Water- shed Society is pleased to provide this handbook, which helps us achieve our vision as well as work to complete our Moose Lake Watershed Management Plan goals. Vision To maintain a healthy and func- tioning Moose Lake Watershed and recognize the importance of living within the capacity of the natural environment as a means of ensuring sustainable environ- mental, economic and social values. Acknowledgments This handbook was created for the Moose Lake Watershed Society by Kellie Nichiporik, with the assis- tance of the staff of the Beaver River Watershed Alliance and reviewed by members of the Moose Lake Watershed Society. Photos by Kellie Nichiporik. Thank you to the Lac La Nonne Enhancement and Protection Association, Waters Edge Resource Group and Lac La Nonne Watershed Stewardship Society for their pioneering accomplishments of the Lac La Nonne and Nakamun Lake Handbooks, upon which this project is based. Thank you to Bill Fox for providing the history of Moose Lake. Thank you to Lakeland Agricultural Research Association and Cows and Fish for providing resources and assistance. -
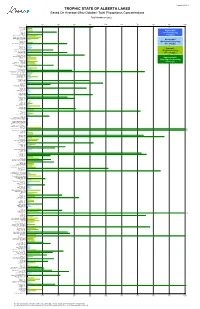
Trophic State of Alberta Lakes Based on Average Total Phosphorus
Created Feb 2013 TROPHIC STATE OF ALBERTA LAKES Based On Average (May-October) Total Phosphorus Concentrations Total Phosphorus (µg/L) 0 100 200 300 400 500 600 700 800 900 1000 * Adamson Lake Alix Lake * Amisk Lake * Angling Lake Oligotrophic * ‡ Antler Lake Arm Lake (Low Productivity) * Astotin Lake (<10 µg/L) * ‡ Athabasca (Lake) - Off Delta Baptiste Lake - North Basin Baptiste Lake - South Basin * ‡ Bare Creek Res. Mesotrophic * ‡ Barrier Lake ‡ Battle Lake (Moderate Productivity) * † Battle River Res. (Forestburg) (10 - 35 µg/L) Beartrap Lake Beauvais Lake Beaver Lake * Bellevue Lake Eutrophic * † Big Lake - East Basin * † Big Lake - West Basin (High Productivity) * Blackfalds Lake (35 - 100 µg/L) * † Blackmud Lake * ‡ Blood Indian Res. Bluet (South Garnier Lake) ‡ Bonnie Lake Hypereutrophic † Borden Lake * ‡ Bourque Lake (Very High Productivity) ‡ Buck Lake (>100 µg/L) Buffalo Lake - Main Basin Buffalo Lake - Secondary Bay * † Buffalo Lake (By Boyle) † Burntstick Lake Calling Lake * † Capt Eyre Lake † Cardinal Lake * ‡ Carolside Res. - Berry Creek Res. † Chain Lakes Res. - North Basin † Chain Lakes Res.- South Basin Chestermere Lake * † Chickakoo Lake * † Chickenhill Lake * Chin Coulee Res. * Clairmont Lake Clear (Barns) Lake Clear Lake ‡ Coal Lake * ‡ Cold Lake - English Bay ‡ Cold Lake - West Side ‡ Cooking Lake † Cow Lake * Crawling Valley Res. Crimson Lake Crowsnest Lake * † Cutbank Lake Dillberry Lake * Driedmeat Lake ‡ Eagle Lake ‡ Elbow Lake Elkwater Lake Ethel Lake * Fawcett Lake * † Fickle Lake * † Figure Eight Lake * Fishing Lake * Flyingshot Lake * Fork Lake * ‡ Fox Lake Res. Frog Lake † Garner Lake Garnier Lake (North) * George Lake * † Ghost Res. - Inside Bay * † Ghost Res. - Inside Breakwater ‡ Ghost Res. - Near Cochrane * Gleniffer Lake (Dickson Res.) * † Glenmore Res. -

Western Grebe Surveys in Alberta 2016
WESTERN GREBE SURVEYS IN ALBERTA 2016 The western grebe has been listed as a Threatened species in Alberta. A recent data compilation shows that there are approximately 250 lakes that have supported western grebes in Alberta. However, information for most lakes is poor and outdate d. Total counts on lakes are rare, breeding status is uncertain, and the location and extent of breeding habitat (emergent vegetation, usually bulrush) is usually unknown. We are seeking your help in gathering more information on western grebe populations in Alberta. If you visit any of the lakes listed below, or know anyone that does, we would appreciate as much detail as you can collect on the presence of western grebes and their habitat. Let us know in advance (if possible) if you are planning on going to any lakes, and when you do, e-mail details of your observations to [email protected]. SURVEY METHODS: Visit a lake between 1 May and 31 August with spotting scope or good binoculars. Surveys can be done from a boat, or vantage point(s) from shore. Report names of surveyors, dates, number of adults seen, and report on the approximate percentage of the lake area that this number represents. Record presence of young birds or nesting colonies, and provide any additional information on presence/location of likely breeding habitat, specific parts of the lake observed, observed threats to birds or habitat (boat traffic, shoreline clearing, pollution, etc.). Please report on findings even if no birds were seen. Lakes on the following page that are flagged with an asterisk (*) were not visited in 2015, and are priority for survey in 2016. -

Status of the Western Grebe (Aechmophorus Occidentalis) in Alberta
Status of the Western Grebe (Aechmophorus occidentalis) in Alberta Alberta Wildlife Status Report No. 60 Status of the Western Grebe (Aechmophorus occidentalis) in Alberta Prepared for: Alberta Sustainable Resource Development (SRD) Alberta Conservation Association (ACA) Prepared by: Jill Yanch This report has been reviewed, revised, and edited prior to publication. It is an SRD/ACA working document that will be revised and updated periodically. Alberta Wildlife Status Report No. 60 June 2006 Published By: i Publication No. T/107 ISBN: 0-7785-4548-2 (Printed Edition) ISBN: 0-7785-4549-0 (On-line Edition) ISSN: 1206-4912 (Printed Edition) ISSN: 1499-4682 (On-line Edition) Series Editors: Sue Peters, Robin Gutsell, Nyree Sharp and Lisa Matthias Illustrations: Brian Huffman Maps: Nicole Hopkins For copies of this report, visit our web site at: http://www.srd.gov.ab.ca/fw/speciesatrisk/ and click on “Detailed Status” OR Contact: Information Centre - Publications Alberta Sustainable Resource Development Main Floor, Great West Life Building 9920 - 108 Street Edmonton, Alberta, Canada T5K 2M4 Telephone: (780) 422-2079 This publication may be cited as: Alberta Sustainable Resource Development and Alberta Conservation Association. 2006. Status of the western grebe (Aechmophorus occidentalis) in Alberta. Alberta Sustainable Resource Development, Wildlife Status Report No. 60, Edmonton, AB. 29 pp. ii PREFACE Every five years, the Fish and Wildlife Division of Alberta Sustainable Resource Development revews the general status of wldlfe speces n Alberta. These overvews, whch have been conducted n 1991 (The Status of Alberta Wildlife), 1996 (The Status of Alberta Wildlife) and 2000 (The General Status of Alberta Wild Species 2000), assgn ndvdual speces “ranks” that reflect the perceived level of risk to populations that occur in the province. -

The Second Report
The Second Report Watershed Stewardship Grant Program (Spring 2006, Fall 2006, Spring 2007) AENV Grant: #06GREA29 Sarah Hipkin & Kevin Wirtanen Grant Administrators 2006-2008 Alberta Stewardship Network Special thanks to: Alberta Environment Members of the Stewardship Grant Committee: Margaret Glasford, ASN Chair / Past Chair and Grant Committee Chair (all grant cycles) Petra Rowell, Alberta Environment (all) Jeff McCammon, Lac La Nonne Watershed and Lake Stewardship Society (all) Shirley Pickering, Highwood Water Management Plan Public Advisory Committee (all) Ernie Ewaschuk and Sarah Primeau, Land Stewardship Centre of Canada & ASN Secretariat Ken Lewis and Dale Chrapko, Alberta Agriculture and Food /AESA Diana Rung, Alberta Conservation Association (all) Kelsey Spicer-Rawe, Alberta Riparian Habitat Management Society (Cows & Fish) (all) Wendy Devent, Stephanie Palechek and Leda Kozak, Oldman Watershed Council Kent Lyle, Sylvan Lake Watershed Stewardship Society (Spring 2006) Frank Vagi, North Saskatchewan Watershed Alliance (Fall 2006) Jacqueline Nelson, ASN Chair (2007/8 on) and Foothills Land Trust (Spring 2008) Jodi Miller, Volunteer Steward (Spring 2008) Additional appreciation is extended to the ASN’s other partnering organizations. The delivery of the range of ASN services is dependent on their ongoing support. 2 CONTENTS The Ripple Reaches Further: The Second Report on the Watershed Stewardship Grant Program ..............................................................................6 Highlights Reach ............................................................................................................................................. -

Watershed Stewardship Grant Program Report
Watershed Stewardship Grant Program Report 2011 An overview of all projects associated with the 2011 Watershed Stewardship Grant Program presented to Alberta Environment and Sustainable Resource Development by Land Stewardship Centre. June 30, 2012 Water It is not only a resource, it is a life source. We all share the responsibility to ensure a healthy, secure and sustainable water supply for our communities, environment, and economy – our quality of life depends on it. As stewards of this precious resource it is our collective duty to ensure: Safe, secure drinking water, Healthy aquatic ecosystems, and Reliable, quality water supplies for a sustainable economy. 2011 Watershed Stewardship Gran t P r o g r a m R e p o r t | 1 Contents Contents ........................................................................................................................................................ 2 Acknowledgements ....................................................................................................................................... 4 2011 Watershed Stewardship Grant Committee Members ..................................................................... 4 2011-2012 Watershed Stewardship Coordinator ..................................................................................... 4 Message from the Committee Chair ............................................................................................................. 5 Highlights of the 2011 Granting Period ....................................................................................................... -

Winter Lakekeepers 2019
Winter LakeKeepers 2019 ALBERTA LAKE MANAGEMENT SOCIETY’S OBJECTIVES ALMS has several objectives, one of which is to collect and interpret water quality data on Alberta Lakes. Equally important is educating lake users about their aquatic environment, encouraging public involvement in lake management, and facilitating cooperation and partnerships between government, industry, the scientific community and lake users. ALMS would like to thank all who express interest in Alberta’s aquatic environments and particularly those who have participated in the LakeKeepers program. These leaders in stewardship give us hope that our water resources will not be the limiting factor in the health of our environment. ACKNOWLEDGEMENTS The LakeKeepers project was made possible with support from Alberta Ecotrust. We would like to thank the volunteers who made this program happen: Jon Pedlan, Ray Walker, Vien and Marielle Lam, Cam and Brittany Kereliuk, Kellie Nichiporik, Steve Hawryliw, and Blake Mills. We would also like to thank the Mighty Peace Watershed Alliance, the Alberta Conservation Association, and the Calling Lake Cottage Association for their assistance with coordinating volunteers and sample shipment. A special thanks to Cerina Lee for developing the LakeKeeper training videos. This report has been prepared by Caitlin Mader, Bradley Peter, Patrick Heney, and Caleb Sinn. EXECUTIVE SUMMARY In 2018, the Alberta Lake Management Society, with financial support from Alberta Ecotrust, piloted the LakeKeepers project. This project was designed to enable stewards to conduct lake monitoring by providing them with training and sampling equipment. In early 2019, this project was expanded to include winter under-ice sampling, with the cooperation of ice anglers. -

Charted Lakes List
LAKE LIST United States and Canada Bull Shoals, Marion (AR), HD Powell, Coconino (AZ), HD Gull, Mono Baxter (AR), Taney (MO), Garfield (UT), Kane (UT), San H. V. Eastman, Madera Ozark (MO) Juan (UT) Harry L. Englebright, Yuba, Chanute, Sharp Saguaro, Maricopa HD Nevada Chicot, Chicot HD Soldier Annex, Coconino Havasu, Mohave (AZ), La Paz HD UNITED STATES Coronado, Saline St. Clair, Pinal (AZ), San Bernardino (CA) Cortez, Garland Sunrise, Apache Hell Hole Reservoir, Placer Cox Creek, Grant Theodore Roosevelt, Gila HD Henshaw, San Diego HD ALABAMA Crown, Izard Topock Marsh, Mohave Hensley, Madera Dardanelle, Pope HD Upper Mary, Coconino Huntington, Fresno De Gray, Clark HD Icehouse Reservior, El Dorado Bankhead, Tuscaloosa HD Indian Creek Reservoir, Barbour County, Barbour De Queen, Sevier CALIFORNIA Alpine Big Creek, Mobile HD DeSoto, Garland Diamond, Izard Indian Valley Reservoir, Lake Catoma, Cullman Isabella, Kern HD Cedar Creek, Franklin Erling, Lafayette Almaden Reservoir, Santa Jackson Meadows Reservoir, Clay County, Clay Fayetteville, Washington Clara Sierra, Nevada Demopolis, Marengo HD Gillham, Howard Almanor, Plumas HD Jenkinson, El Dorado Gantt, Covington HD Greers Ferry, Cleburne HD Amador, Amador HD Greeson, Pike HD Jennings, San Diego Guntersville, Marshall HD Antelope, Plumas Hamilton, Garland HD Kaweah, Tulare HD H. Neely Henry, Calhoun, St. HD Arrowhead, Crow Wing HD Lake of the Pines, Nevada Clair, Etowah Hinkle, Scott Barrett, San Diego Lewiston, Trinity Holt Reservoir, Tuscaloosa HD Maumelle, Pulaski HD Bear Reservoir,