Subtype-Specific and Co-Occurring Genetic Alterations in B-Cell Non
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BCL7A As a Novel Prognostic Biomarker for Glioma Patients
BCL7A as a novel prognostic biomarker for glioma patients Junhui Liu ( [email protected] ) Renmin Hospital of Wuhan University: Wuhan University Renmin Hospital Lun Gao Renmin Hospital of Wuhan University: Wuhan University Renmin Hospital Baowei Ji Renmin Hospital of Wuhan University: Wuhan University Renmin Hospital Rongxin Geng Renmin Hospital of Wuhan University: Wuhan University Renmin Hospital Jing Chen Renmin Hospital of Wuhan University: Wuhan University Renmin Hospital Xiang Tao Renmin Hospital of Wuhan University: Wuhan University Renmin Hospital Qiang Cai Renmin Hospital of Wuhan University: Wuhan University Renmin Hospital Zhibiao Chen Renmin Hospital of Wuhan University: Wuhan University Renmin Hospital Research Article Keywords: BCL7 family, glioma, prognosis, immune, Temozolomide Posted Date: April 21st, 2021 DOI: https://doi.org/10.21203/rs.3.rs-390165/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published at Journal of Translational Medicine on August 6th, 2021. See the published version at https://doi.org/10.1186/s12967-021-03003-0. Page 1/24 Abstract Background: Glioma is the most common primary brain tumor and represents one of the most aggressive and lethal types of human cancer. BCL7 family has been found in several cancer types and could be involved in tumor progression. While the role of BCL7 family in human glioma has remained to be elucidated. Methods: Paran-embedded tumor samples were obtained to detect BCL7 expression by performing in glioma. Data (including normalized gene expression and corresponding clinical data) were obtained from Gliovis, CGGA, GEO, cBioportal and Oncomine and were used to investigate BCL7 genes expression in glioma. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Integrating Genomic Alterations in Diffuse Large B-Cell Lymphoma Identifies New Relevant Pathways and Potential Therapeutic Targets
OPEN Leukemia (2018) 32, 675–684 www.nature.com/leu ORIGINAL ARTICLE Integrating genomic alterations in diffuse large B-cell lymphoma identifies new relevant pathways and potential therapeutic targets K Karube1,2, A Enjuanes1,3, I Dlouhy1, P Jares1,3, D Martin-Garcia1,3, F Nadeu1,3, GR Ordóñez4, J Rovira1, G Clot1,3, C Royo1, A Navarro1,3, B Gonzalez-Farre1,3, A Vaghefi1, G Castellano1, C Rubio-Perez5, D Tamborero5, J Briones6, A Salar7, JM Sancho8, S Mercadal9, E Gonzalez-Barca9, L Escoda10, H Miyoshi11, K Ohshima11, K Miyawaki12, K Kato12, K Akashi12, A Mozos13, L Colomo1,7, M Alcoceba3,14, A Valera1, A Carrió1,3, D Costa1,3, N Lopez-Bigas5,15, R Schmitz16, LM Staudt16, I Salaverria1,3, A López-Guillermo1,3 and E Campo1,3 Genome studies of diffuse large B-cell lymphoma (DLBCL) have revealed a large number of somatic mutations and structural alterations. However, the clinical significance of these alterations is still not well defined. In this study, we have integrated the analysis of targeted next-generation sequencing of 106 genes and genomic copy number alterations (CNA) in 150 DLBCL. The clinically significant findings were validated in an independent cohort of 111 patients. Germinal center B-cell and activated B-cell DLBCL had a differential profile of mutations, altered pathogenic pathways and CNA. Mutations in genes of the NOTCH pathway and tumor suppressor genes (TP53/CDKN2A), but not individual genes, conferred an unfavorable prognosis, confirmed in the independent validation cohort. A gene expression profiling analysis showed that tumors with NOTCH pathway mutations had a significant modulation of downstream target genes, emphasizing the relevance of this pathway in DLBCL. -

Pathognomonic and Epistatic Genetic Alterations in B-Cell Non-Hodgkin
bioRxiv preprint doi: https://doi.org/10.1101/674259; this version posted June 19, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Pathognomonic and epistatic genetic alterations in 2 B-cell non-Hodgkin lymphoma 3 4 Man Chun John Ma1¥, Saber Tadros1¥, Alyssa Bouska2, Tayla B. Heavican2, Haopeng Yang1, 5 Qing Deng1, Dalia Moore3, Ariz Akhter4, Keenan Hartert3, Neeraj Jain1, Jordan Showell1, 6 Sreejoyee Ghosh1, Lesley Street5, Marta Davidson5, Christopher Carey6, Joshua Tobin7, 7 Deepak Perumal8, Julie M. Vose9, Matthew A. Lunning9, Aliyah R. Sohani10, Benjamin J. 8 Chen11, Shannon Buckley12, Loretta J. Nastoupil1, R. Eric Davis1, Jason R. Westin1, Nathan H. 9 Fowler1, Samir Parekh8, Maher K. Gandhi7, Sattva S. Neelapu1, Douglas Stewart5, Javeed 10 Iqbal2, Timothy Greiner2, Scott J. Rodig13, Adnan Mansoor5, Michael R. Green1,14,15* 11 1Department of Lymphoma and Myeloma, Division of Cancer Medicine, The University of Texas MD 12 Anderson Cancer Center, Houston, TX, USA; 2Department of Pathology and Microbiology, University of 13 Nebraska Medical Center, Omaha, NE, USA; 3Eppley Institute for Research in Cancer and Allied 14 Diseases, University of Nebraska Medical Center, Omaha, NE, USA; 4Department of Pathology and 15 Laboratory Medicine, University of Calgary, Calgary, AB, Canada; 5Section of Hematology, Department of 16 Medicine, University -

BCL7A Antibody Cat
BCL7A Antibody Cat. No.: 45-325 BCL7A Antibody 45-325 Immunofluorescence analysis of 45-325 Flow cytometric analysis of paraformaldehyde paraformaldehyde fixed U2OS cells, permeabilized with fixed Daudi cells (blue line), permeabilized with 0.5% 0.15% Triton. Primary incubation 1hr (10ug/ml) followed Triton. Primary incubation 1hr (10ug/ml) followed by Alexa by Alexa Fluor 488 secondary antibody (2ug/ml), showing Fluor 488 secondary antibody (1ug/ml). IgG control: nuclear staining. Actin filaments were stained wit Unimmunized goat IgG (black line) fo Specifications HOST SPECIES: Goat SPECIES REACTIVITY: Human HOMOLOGY: Expected Species Reactivity based on sequence homology: Mouse, Rat, Dog, Cow, Pig IMMUNOGEN: The immunogen for this antibody is: C-MKLEASQQNSEEM TESTED APPLICATIONS: ELISA, Flow, IF, WB October 2, 2021 1 https://www.prosci-inc.com/bcl7a-antibody-45-325.html Peptide ELISA: antibody detection limit dilution 1:4000.Western Blot:Approx 26kDa band observed in Human Lymph Node lysates (calculated MW of 25.0kDa according to NP_066273.1). Recommended concentration: 1-3ug/ml. Primary incubation 1 hour at APPLICATIONS: room temperature.Immunofluorescence: Strong expression of the protein seen in the nuclei of U2OS cells. Recommended concentration: 10ug/ml. Flow Cytometry: Flow cytometric analysis of Daudi cells. Recommended concentration: 10ug/ml. This antibody is expected to recognize both reported isoforms (NP_066273.1; SPECIFICITY: NP_001019979.1). POSITIVE CONTROL: 1) Cat. No. 1201 - HeLa Cell Lysate Properties Purified from goat serum by ammonium sulphate precipitation followed by antigen PURIFICATION: affinity chromatography using the immunizing peptide. CLONALITY: Polyclonal CONJUGATE: Unconjugated PHYSICAL STATE: Liquid Supplied at 0.5 mg/ml in Tris saline, 0.02% sodium azide, pH7.3 with 0.5% bovine serum BUFFER: albumin. -

Characterization of the ING1 Candidate Tumor Suppressor Gene in Breast Cancer Cells
University of Calgary PRISM: University of Calgary's Digital Repository Graduate Studies Legacy Theses 2001 Characterization of the ING1 candidate tumor suppressor gene in breast cancer cells Nelson, Rebecca Nelson, R. (2001). Characterization of the ING1 candidate tumor suppressor gene in breast cancer cells (Unpublished master's thesis). University of Calgary, Calgary, AB. doi:10.11575/PRISM/11964 http://hdl.handle.net/1880/41031 master thesis University of Calgary graduate students retain copyright ownership and moral rights for their thesis. You may use this material in any way that is permitted by the Copyright Act or through licensing that has been assigned to the document. For uses that are not allowable under copyright legislation or licensing, you are required to seek permission. Downloaded from PRISM: https://prism.ucalgary.ca The author of this thesis has granted the University of Calgary a non-exclusive license to reproduce and distribute copies of this thesis to users of the University of Calgary Archives. Copyright remains with the author. Theses and dissertations available in the University of Calgary Institutional Repository are solely for the purpose of private study and research. They may not be copied or reproduced, except as permitted by copyright laws, without written authority of the copyright owner. Any commercial use or publication is strictly prohibited. The original Partial Copyright License attesting to these terms and signed by the author of this thesis may be found in the original print version of the thesis, held by the University of Calgary Archives. The thesis approval page signed by the examining committee may also be found in the original print version of the thesis held in the University of Calgary Archives. -

ANALYSIS Doi:10.1038/Nature14663
ANALYSIS doi:10.1038/nature14663 Universal allosteric mechanism for Ga activation by GPCRs Tilman Flock1, Charles N. J. Ravarani1*, Dawei Sun2,3*, A. J. Venkatakrishnan1{, Melis Kayikci1, Christopher G. Tate1, Dmitry B. Veprintsev2,3 & M. Madan Babu1 G protein-coupled receptors (GPCRs) allosterically activate heterotrimeric G proteins and trigger GDP release. Given that there are 800 human GPCRs and 16 different Ga genes, this raises the question of whether a universal allosteric mechanism governs Ga activation. Here we show that different GPCRs interact with and activate Ga proteins through a highly conserved mechanism. Comparison of Ga with the small G protein Ras reveals how the evolution of short segments that undergo disorder-to-order transitions can decouple regions important for allosteric activation from receptor binding specificity. This might explain how the GPCR–Ga system diversified rapidly, while conserving the allosteric activation mechanism. proteins bind guanine nucleotides and act as molecular switches almost 30 A˚ away from the GDP binding region5 and allosterically trig- in a number of signalling pathways by interconverting between ger GDP release to activate them. 1,2 G a GDP-bound inactive and a GTP-bound active state . They The high-resolution structure of the Gas-bound b2-adrenergic recep- 3 5 consist of two major classes: monomeric small G proteins and hetero- tor (b2AR) provided crucial insights into the receptor–G protein inter- trimeric G proteins4. While small G proteins and the a-subunit (Ga)of face and conformational changes in Ga upon receptor binding6,7. Recent 6 8 heterotrimeric G proteins both contain a GTPase domain (G-domain), studies described dynamic regions in Gas and Gai , the importance of ˚ Ga contains an additional helical domain (H-domain) and also forms a displacement of helix 5 (H5) of Gas and Gat by up to 6 A into the complex with the Gb and Gc subunits. -

Analysis of the Indacaterol-Regulated Transcriptome in Human Airway
Supplemental material to this article can be found at: http://jpet.aspetjournals.org/content/suppl/2018/04/13/jpet.118.249292.DC1 1521-0103/366/1/220–236$35.00 https://doi.org/10.1124/jpet.118.249292 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS J Pharmacol Exp Ther 366:220–236, July 2018 Copyright ª 2018 by The American Society for Pharmacology and Experimental Therapeutics Analysis of the Indacaterol-Regulated Transcriptome in Human Airway Epithelial Cells Implicates Gene Expression Changes in the s Adverse and Therapeutic Effects of b2-Adrenoceptor Agonists Dong Yan, Omar Hamed, Taruna Joshi,1 Mahmoud M. Mostafa, Kyla C. Jamieson, Radhika Joshi, Robert Newton, and Mark A. Giembycz Departments of Physiology and Pharmacology (D.Y., O.H., T.J., K.C.J., R.J., M.A.G.) and Cell Biology and Anatomy (M.M.M., R.N.), Snyder Institute for Chronic Diseases, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada Received March 22, 2018; accepted April 11, 2018 Downloaded from ABSTRACT The contribution of gene expression changes to the adverse and activity, and positive regulation of neutrophil chemotaxis. The therapeutic effects of b2-adrenoceptor agonists in asthma was general enriched GO term extracellular space was also associ- investigated using human airway epithelial cells as a therapeu- ated with indacaterol-induced genes, and many of those, in- tically relevant target. Operational model-fitting established that cluding CRISPLD2, DMBT1, GAS1, and SOCS3, have putative jpet.aspetjournals.org the long-acting b2-adrenoceptor agonists (LABA) indacaterol, anti-inflammatory, antibacterial, and/or antiviral activity. Numer- salmeterol, formoterol, and picumeterol were full agonists on ous indacaterol-regulated genes were also induced or repressed BEAS-2B cells transfected with a cAMP-response element in BEAS-2B cells and human primary bronchial epithelial cells by reporter but differed in efficacy (indacaterol $ formoterol . -

ING1 Induces Apoptosis Through Direct Effects at the Mitochondria
Citation: Cell Death and Disease (2013) 4, e837; doi:10.1038/cddis.2013.398 & 2013 Macmillan Publishers Limited All rights reserved 2041-4889/13 www.nature.com/cddis Corrigendum ING1 induces apoptosis through direct effects at the mitochondria P Bose, S Thakur, S Thalappilly, BY Ahn, S Satpathy, X Feng, K Suzuki, SW Kim and K Riabowol The ING family of tumor suppressors acts as readers and writers of the histone epigenetic code, affecting DNA repair, chromatin remodeling, cellular senescence, cell cycle regulation and apoptosis. The best characterized member of the ING family, ING1, interacts with the proliferating cell nuclear antigen (PCNA) in a UV-inducible manner. ING1 also interacts with members of the 14-3-3 family leading to its cytoplasmic relocalization. Overexpression of ING1 enhances expression of the Bax gene and was reported to alter mitochondrial membrane potential in a p53-dependent manner. Here we show that ING1 translocates to the mitochondria of primary fibroblasts and established epithelial cell lines in response to apoptosis inducing stimuli, independent of the cellular p53 status. The ability of ING1 to induce apoptosis in various breast cancer cell lines correlates well with its degree of translocation to the mitochondria after UV treatment. Endogenous ING1 protein specifically interacts with the pro-apoptotic BCL2 family member BAX, and colocalizes with BAX in a UV-inducible manner. Ectopic expression of a mitochondria-targeted ING1 construct is more proficient in inducing apoptosis than the wild type ING1 protein. Bioinformatic analysis of the yeast interactome indicates that yeast ING proteins interact with 64 mitochondrial proteins. Also, sequence analysis of ING1 reveals the presence of a BH3-like domain. -

Genomic Analyses of PMBL Reveal New Drivers and Mechanisms of Sensitivity to PD-1 Blockade
Regular Article LYMPHOID NEOPLASIA Genomic analyses of PMBL reveal new drivers and mechanisms of sensitivity to PD-1 blockade Bjoern Chapuy,1,2,* Chip Stewart,3,* Andrew J. Dunford,3,* Jaegil Kim,3 Kirsty Wienand,1 Atanas Kamburov,3 Gabriel K. Griffin,4 Pei-Hsuan Chen,4 Ana Lako,4 Robert A. Redd,5 Claire M. Cote,1 Matthew D. Ducar,6 Aaron R. Thorner,6 Scott J. Rodig,4 Gad Getz,3,7,8,† Downloaded from https://ashpublications.org/blood/article-pdf/134/26/2369/1549702/bloodbld2019002067.pdf by Margaret Shipp on 30 December 2019 and Margaret A. Shipp1,† 1Department of Medical Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA; 2Department of Hematology and Oncology, University Medical Center Gottingen,¨ Gottingen,¨ Germany; 3Broad Institute of Harvard and Massachusetts Institute of Technology, Cambridge, MA; 4Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 5Department of Data Sciences and 6Center for Cancer Genome Discovery, Dana-Farber Cancer Institute, Boston, MA; and 7Department of Pathology and 8Center for Cancer Research, Massachusetts General Hospital, Harvard Medical School, Boston, MA KEY POINTS Primary mediastinal large B-cell lymphomas (PMBLs) are aggressive tumors that typically present as large mediastinal masses in young women. PMBLs share clinical, transcriptional, l Comprehensive genomic analyses of and molecular features with classical Hodgkin lymphoma (cHL), including constitutive PMBL reveal new activation of nuclear factor kB (NF-kB), JAK/STAT signaling, and programmed cell death genetic drivers such protein 1 (PD-1)–mediated immune evasion. The demonstrated efficacy of PD-1 blockade in as ZNF217. relapsed/refractory PMBLs led to recent approval by the US Food and Drug Administration l High mutational and underscored the importance of characterizing targetable genetic vulnerabilities in this burden, MSI, and disease. -

Transcriptome Analysis of Human Diabetic Kidney Disease
ORIGINAL ARTICLE Transcriptome Analysis of Human Diabetic Kidney Disease Karolina I. Woroniecka,1 Ae Seo Deok Park,1 Davoud Mohtat,2 David B. Thomas,3 James M. Pullman,4 and Katalin Susztak1,5 OBJECTIVE—Diabetic kidney disease (DKD) is the single cases, mild and then moderate mesangial expansion can be leading cause of kidney failure in the U.S., for which a cure has observed. In general, diabetic kidney disease (DKD) is not yet been found. The aim of our study was to provide an considered a nonimmune-mediated degenerative disease unbiased catalog of gene-expression changes in human diabetic of the glomerulus; however, it has long been noted that kidney biopsy samples. complement and immunoglobulins sometimes can be de- — tected in diseased glomeruli, although their role and sig- RESEARCH DESIGN AND METHODS Affymetrix expression fi arrays were used to identify differentially regulated transcripts in ni cance is not clear (4). 44 microdissected human kidney samples. The DKD samples were The understanding of DKD has been challenged by multi- significant for their racial diversity and decreased glomerular ple issues. First, the diagnosis of DKD usually is made using filtration rate (~20–30 mL/min). Stringent statistical analysis, using clinical criteria, and kidney biopsy often is not performed. the Benjamini-Hochberg corrected two-tailed t test, was used to According to current clinical practice, the development of identify differentially expressed transcripts in control and diseased albuminuria in patients with diabetes is sufficient to make the glomeruli and tubuli. Two different Web-based algorithms were fi diagnosis of DKD (5). We do not understand the correlation used to de ne differentially regulated pathways. -
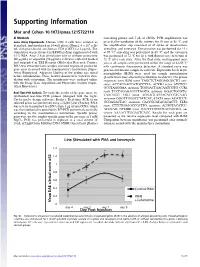
Supporting Information
Supporting Information Mor and Cohen 10.1073/pnas.1215722110 SI Methods containing primer and 5 μL of cDNA. PCR amplification was Gene Array Experiments. Human CD4 T cells were isolated as preceded by incubation of the mixture for 10 min at 95 °C, and described, and incubated in 24-well plates (Nunc), 4 × 106 cells/ the amplification step consisted of 45 cycles of denaturation, mL with plate-bound anti-human CD3 (OKT3) at 2 μg/mL. The annealing, and extension. Denaturation was performed for 15 s stimulation was performed in RPMI medium supplemented with at 95 °C; annealing was performed in 60 °C; and the extension 0.1% BSA. After 2 h of stimulation with or without cefuroxime was performed at 72 °C for 20 s, with fluorescence detection at (50 μg/mL) or ampicillin (50 μg/mL), cells were collected washed 72 °C after each cycle. After the final cycle, melting-point anal- and suspended in TRI Reagent (Molecular Research Center). yses of all samples were performed within the range of 62–95 °C RNA was extracted from samples and used to prepare probes for with continuous fluorescence detection. A standard curve was gene array in accord with the manufacturer’s instructions (Super- generated from one sample in each run. Expression levels of β2- Array Bioscience). Adequate labeling of the probes was tested microglobulin (B2M) were used for sample normalization before hybridization. Three healthy donors were tested in stim- (β-actin levels were affected by cefuroxime treatment). The primer ulation with cefuroxime. The membranes were analyzed online sequences were B2M sense TAGCTCTAGGAGGGCTG anti- with the Image Data Acquisition and Expression Analysis (Super- sense ACCACAACCATGCCTTA; ACVR2 sense ATCTCC- Array Bioscience).