Loss of ZBTB20 Impairs Circadian Output and Leads to Unimodal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model
Downloaded from http://www.jimmunol.org/ by guest on September 25, 2021 T + is online at: average * The Journal of Immunology , 34 of which you can access for free at: 2016; 197:1477-1488; Prepublished online 1 July from submission to initial decision 4 weeks from acceptance to publication 2016; doi: 10.4049/jimmunol.1600589 http://www.jimmunol.org/content/197/4/1477 Molecular Profile of Tumor-Specific CD8 Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. Waugh, Sonia M. Leach, Brandon L. Moore, Tullia C. Bruno, Jonathan D. Buhrman and Jill E. Slansky J Immunol cites 95 articles Submit online. Every submission reviewed by practicing scientists ? is published twice each month by Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html http://www.jimmunol.org/content/suppl/2016/07/01/jimmunol.160058 9.DCSupplemental This article http://www.jimmunol.org/content/197/4/1477.full#ref-list-1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material References Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of September 25, 2021. The Journal of Immunology Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. -

Edinburgh Research Explorer
Edinburgh Research Explorer International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list Citation for published version: Davenport, AP, Alexander, SPH, Sharman, JL, Pawson, AJ, Benson, HE, Monaghan, AE, Liew, WC, Mpamhanga, CP, Bonner, TI, Neubig, RR, Pin, JP, Spedding, M & Harmar, AJ 2013, 'International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands', Pharmacological reviews, vol. 65, no. 3, pp. 967-86. https://doi.org/10.1124/pr.112.007179 Digital Object Identifier (DOI): 10.1124/pr.112.007179 Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher's PDF, also known as Version of record Published In: Pharmacological reviews Publisher Rights Statement: U.S. Government work not protected by U.S. copyright General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 02. Oct. 2021 1521-0081/65/3/967–986$25.00 http://dx.doi.org/10.1124/pr.112.007179 PHARMACOLOGICAL REVIEWS Pharmacol Rev 65:967–986, July 2013 U.S. -

G Protein-Coupled Receptors in Stem Cell Maintenance and Somatic Reprogramming to Pluripotent Or Cancer Stem Cells
BMB Reports - Manuscript Submission Manuscript Draft Manuscript Number: BMB-14-250 Title: G protein-coupled receptors in stem cell maintenance and somatic reprogramming to pluripotent or cancer stem cells Article Type: Mini Review Keywords: G protein-coupled receptors; stem cell maintenance; somatic reprogramming; cancer stem cells; pluripotent stem cell Corresponding Author: Ssang-Goo Cho Authors: Ssang-Goo Cho1,*, Hye Yeon Choi1, Subbroto Kumar Saha1, Kyeongseok Kim1, Sangsu Kim1, Gwang-Mo Yang1, BongWoo Kim1, Jin-hoi Kim1 Institution: 1Department of Animal Biotechnology, Animal Resources Research Center, and Incurable Disease Animal Model and Stem Cell Institute (IDASI), Konkuk University, 120 Neungdong-ro, Gwangjin-gu, Seoul 143-701, Republic of Korea, UNCORRECTED PROOF 1 G protein-coupled receptors in stem cell maintenance and somatic reprogramming to 2 pluripotent or cancer stem cells 3 4 Hye Yeon Choi, Subbroto Kumar Saha, Kyeongseok Kim, Sangsu Kim, Gwang-Mo 5 Yang, BongWoo Kim, Jin-hoi Kim, and Ssang-Goo Cho 6 7 Department of Animal Biotechnology, Animal Resources Research Center, and 8 Incurable Disease Animal Model and Stem Cell Institute (IDASI), Konkuk University, 9 120 Neungdong-ro, Gwangjin-gu, Seoul 143-701, Republic of Korea 10 * 11 Address correspondence to Ssang-Goo Cho, Department of Animal Biotechnology and 12 Animal Resources Research Center. Konkuk University, 120 Neungdong-ro, Gwangjin- 13 gu, Seoul 143-701, Republic of Korea. Tel: 82-2-450-4207, Fax: 82-2-450-1044, E-mail: 14 [email protected] 15 16 17 18 19 1 UNCORRECTED PROOF 20 Abstract 21 The G protein-coupled receptors (GPCRs) compose the third largest gene family in the 22 human genome, representing more than 800 distinct genes and 3–5% of the human genome. -

Health-Relevant Phenotypes in the Offspring of Mice Given CAR Activators Prior to Pregnancy S
Supplemental material to this article can be found at: http://dmd.aspetjournals.org/content/suppl/2018/08/28/dmd.118.082925.DC1 1521-009X/46/11/1827–1835$35.00 https://doi.org/10.1124/dmd.118.082925 DRUG METABOLISM AND DISPOSITION Drug Metab Dispos 46:1827–1835, November 2018 Copyright ª 2018 by The American Society for Pharmacology and Experimental Therapeutics Health-Relevant Phenotypes in the Offspring of Mice Given CAR Activators Prior to Pregnancy s Karin Dietrich, Jan Baumgart, Leonid Eshkind, Lea Reuter, Ute Gödtel-Armbrust, Elke Butt, Michael Musheev, Federico Marini, Piyush More, Tanja Grosser, Christof Niehrs, Leszek Wojnowski, and Marianne Mathäs Department of Pharmacology (K.D., L.R., U.G.-A., P.M., T.G., L.W., M.Ma.) and Institute of Medical Biostatistics, Epidemiology and Informatics (F.M.), University Medical Center Mainz, Mainz, Germany; Translational Animal Research Center (J.B., L.E.), University Medical Center, Johannes Gutenberg University Mainz, Mainz, Germany; Institute of Experimental Biomedicine II, University Hospital Würzburg, Würzburg, Germany (E.B.); Institute of Molecular Biology, Mainz, Germany (M.Mu., C.N.); and Division of Molecular Embryology, German Cancer Research Center (DKFZ-ZMBH Alliance), Heidelberg, Germany (C.N.) Received June 6, 2018; accepted August 22, 2018 Downloaded from ABSTRACT Hepatic induction in response to drugs and environmental chem- analyses. The important environmental pollutant PCB153 acti- icals affects drug therapies and energy metabolism. We investigated vated CAR in the F1 generation in a manner similar to TCPOBOP. whether the induction is transmitted to the offspring. We injected Our findings indicate that chemicals accumulating and persisting 3-day- and 6-week-old F0 female mice with TCPOBOP, an activator in adipose tissue may exert liver-mediated, health-relevant effects dmd.aspetjournals.org of the nuclear receptor constitutive androstane receptor (CAR, on F1 offspring simply via physical transmission in milk. -

A Wheel of Time: the Circadian Clock, Nuclear Receptors, and Physiology
Downloaded from genesdev.cshlp.org on September 29, 2021 - Published by Cold Spring Harbor Laboratory Press PERSPECTIVE A wheel of time: the circadian clock, nuclear receptors, and physiology Xiaoyong Yang1 Program in Integrative Cell Signaling and Neurobiology of Metabolism, Section of Comparative Medicine, Department of Cellular and Molecular Physiology, Yale University School of Medicine, New Haven, Connecticut 06519, USA It is a long-standing view that the circadian clock func- The rhythmic production and circulation of many tions to proactively align internal physiology with the hormones and metabolites within the endocrine system 24-h rotation of the earth. Recent studies, including one is instrumental in regulating regular physiological pro- by Schmutz and colleagues (pp. 345–357) in the February cesses such as reproduction, blood pressure, and metabo- 15, 2010, issue of Genes & Development, delineate strik- lism. Levels of circulating estrogen and progesterone ingly complex connections between molecular clocks and fluctuate with the menstrual cycle, which in turn affect nuclear receptor signaling pathways, implying the exis- circadian rhythms in women (Shechter and Boivin 2010). tence of a large-scale circadian regulatory network co- In parallel with a diurnal rhythm in circulating adrenocor- ordinating a diverse array of physiological processes to ticotropic hormone, secretion of glucocorticoids and aldo- maintain dynamic homeostasis. sterone from the adrenal gland rises before awakening (Weitzman 1976). Glucocorticoids boost energy produc- tion, and aldosterone increases blood pressure, together gearing up the body for the activity phase. Similarly, Light from the sun sustains life on earth. The 24-h plasma levels of thyroid-stimulating hormone and triiodo- rotation of the earth exposes a vast number of plants thyronine have a synchronous diurnal rhythm (Russell and animals to the light/dark cycle. -

Melatonin Synthesis and Clock Gene Regulation in the Pineal Organ Of
General and Comparative Endocrinology 279 (2019) 27–34 Contents lists available at ScienceDirect General and Comparative Endocrinology journal homepage: www.elsevier.com/locate/ygcen Review article Melatonin synthesis and clock gene regulation in the pineal organ of teleost fish compared to mammals: Similarities and differences T ⁎ Saurav Saha, Kshetrimayum Manisana Singh, Braj Bansh Prasad Gupta Environmental Endocrinology Laboratory, Department of Zoology, North-Eastern Hill University, Shillong 793022, India ARTICLE INFO ABSTRACT Keywords: The pineal organ of all vertebrates synthesizes and secretes melatonin in a rhythmic manner due to the circadian Aanat gene rhythm in the activity of arylalkylamine N-acetyltransferase (AANAT) – the rate-limiting enzyme in melatonin Circadian rhythm synthesis pathway. Nighttime increase in AANAT activity and melatonin synthesis depends on increased ex- Clock genes pression of aanat gene (a clock-controlled gene) and/or post-translation modification of AANAT protein. In Melatonin synthesis mammalian and avian species, only one aanat gene is expressed. However, three aanat genes (aanat1a, aanat1b, Pineal organ and aanat2) are reported in fish species. While aanat1a and aanat1b genes are expressed in the fish retina, the Photoperiod fi Temperature nervous system and other peripheral tissues, aanat2 gene is expressed exclusively in the sh pineal organ. Clock genes form molecular components of the clockwork, which regulates clock-controlled genes like aanat gene. All core clock genes (i.e., clock, bmal1, per1, per2, per3, cry1 and cry2) and aanat2 gene (a clock-controlled gene) are expressed in the pineal organ of several fish species. There is a large body of information on regulation of clock genes, aanat gene and melatonin synthesis in the mammalian pineal gland. -
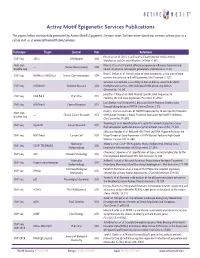
Active Motif Epigenetic Services Publications the Papers Below Contain Data Generated by Active Motif’S Epigenetic Services Team
Active Motif Epigenetic Services Publications The papers below contain data generated by Active Motif’s Epigenetic Services team. To learn more about our services, please give us a call or visit us at www.activemotif.com/services. Technique Target Journal Year Reference Erin Stashi et al. SRC-2 Is an Essential Coactivator for Orchestrating ChIP-Seq SRC-2 Cell Reports 2014 Metabolism and Circadian Rhythm. Cell Rep. 6: 663 Next-Gen Brian G. Dias et al. Parental olfactory experience influences behavior and — Nature Neuroscience 2014 Bisulfite Seq neural structure in subsequent generations. Nat Neurosci. 17: 89 Brian C. Belyea et al. Identification of renin progenitors in the mouse bone ChIP-Seq H3K4me3, H3K27me3 Nature Communications 2014 marrow that give rise to B-cell leukaemia. Nat Commun. 5: 3273 Variation in chromatin accessibility in human kidney cancer links H3K36 ChIP-Seq H3K36me3 Genome Research 2014 methyltransferase loss with widespread RNA processing defects. Genome Res. 24: 241 Jonathan P. Riley et al. PARP-14 Binds Specific DNA Sequences to ChIP-Seq RNA Pol II PLoS One 2013 Promote Th2 Cell Gene Expression. PLoS One. 8: e83127 Lynn Bjerke et al. Histone H3.3 Mutations Drive Pediatric Glioblastoma ChIP-Seq H3K36me3 Cancer Discovery 2013 through Upregulation of MYCN. Cancer Discov. 3: 512 David S. Shames et al. Loss of NAPRT1 Expression by Tumor-Specific Promoter Next-Gen — Clinical Cancer Research 2013 Methylation Provides a Novel Predictive Biomarker for NAMPT Inhibitors. Bisulfite Seq Clin Cancer Res. 19: 6912 Xiaoming Ju et al. Identification of a cyclin D1 network in prostate cancer ChIP-Seq Cyclin D1 Cancer Research 2013 that antagonizes epithelial-mesenchymal restraint. -

Role of the Nuclear Receptor Rev-Erb Alpha in Circadian Food Anticipation and Metabolism Julien Delezie
Role of the nuclear receptor Rev-erb alpha in circadian food anticipation and metabolism Julien Delezie To cite this version: Julien Delezie. Role of the nuclear receptor Rev-erb alpha in circadian food anticipation and metabolism. Neurobiology. Université de Strasbourg, 2012. English. NNT : 2012STRAJ018. tel- 00801656 HAL Id: tel-00801656 https://tel.archives-ouvertes.fr/tel-00801656 Submitted on 10 Apr 2013 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITÉ DE STRASBOURG ÉCOLE DOCTORALE DES SCIENCES DE LA VIE ET DE LA SANTE CNRS UPR 3212 · Institut des Neurosciences Cellulaires et Intégratives THÈSE présentée par : Julien DELEZIE soutenue le : 29 juin 2012 pour obtenir le grade de : Docteur de l’université de Strasbourg Discipline/ Spécialité : Neurosciences Rôle du récepteur nucléaire Rev-erbα dans les mécanismes d’anticipation des repas et le métabolisme THÈSE dirigée par : M CHALLET Etienne Directeur de recherche, université de Strasbourg RAPPORTEURS : M PFRIEGER Frank Directeur de recherche, université de Strasbourg M KALSBEEK Andries -

Correlation Between Circadian Gene Variants and Serum Levels of Sex Steroids and Insulin-Like Growth Factor-I
3268 Correlation between Circadian Gene Variants and Serum Levels of Sex Steroids and Insulin-like Growth Factor-I Lisa W. Chu,1,2 Yong Zhu,3 Kai Yu,1 Tongzhang Zheng,3 Anand P. Chokkalingam,4 Frank Z. Stanczyk,5 Yu-Tang Gao,6 and Ann W. Hsing1 1Division of Cancer Epidemiology and Genetics and 2Cancer Prevention Fellowship Program, Office of Preventive Oncology, National Cancer Institute, NIH, Bethesda, Maryland; 3Department of Epidemiology and Public Health, Yale University School of Medicine, New Haven, Connecticut; 4Division of Epidemiology, School of Public Health, University of California at Berkeley, Berkeley, California; 5Department of Obstetrics and Gynecology, Keck School of Medicine, University of Southern California, Los Angeles, California; and 6Department of Epidemiology, Shanghai Cancer Institute, Shanghai, China Abstract A variety of biological processes, including steroid the GG genotype. In addition, the PER1 variant was hormone secretion, have circadian rhythms, which are associated with higher serum levels of sex hormone- P influenced by nine known circadian genes. Previously, binding globulin levels ( trend = 0.03), decreasing we reported that certain variants in circadian genes 5A-androstane-3A,17B-diol glucuronide levels P P were associated with risk for prostate cancer. To pro- ( trend = 0.02), and decreasing IGFBP3 levels ( trend = vide some biological insight into these findings, we 0.05). Furthermore, the CSNK1E variant C allele was examined the relationship of five variants of circadian associated with higher -

G Protein-Coupled Receptors
S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein-coupled receptors. British Journal of Pharmacology (2015) 172, 5744–5869 THE CONCISE GUIDE TO PHARMACOLOGY 2015/16: G protein-coupled receptors Stephen PH Alexander1, Anthony P Davenport2, Eamonn Kelly3, Neil Marrion3, John A Peters4, Helen E Benson5, Elena Faccenda5, Adam J Pawson5, Joanna L Sharman5, Christopher Southan5, Jamie A Davies5 and CGTP Collaborators 1School of Biomedical Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK, 2Clinical Pharmacology Unit, University of Cambridge, Cambridge, CB2 0QQ, UK, 3School of Physiology and Pharmacology, University of Bristol, Bristol, BS8 1TD, UK, 4Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK, 5Centre for Integrative Physiology, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2015/16 provides concise overviews of the key properties of over 1750 human drug targets with their pharmacology, plus links to an open access knowledgebase of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. The full contents can be found at http://onlinelibrary.wiley.com/doi/ 10.1111/bph.13348/full. G protein-coupled receptors are one of the eight major pharmacological targets into which the Guide is divided, with the others being: ligand-gated ion channels, voltage-gated ion channels, other ion channels, nuclear hormone receptors, catalytic receptors, enzymes and transporters. These are presented with nomenclature guidance and summary information on the best available pharmacological tools, alongside key references and suggestions for further reading. -

Biased Signaling of G Protein Coupled Receptors (Gpcrs): Molecular Determinants of GPCR/Transducer Selectivity and Therapeutic Potential
Pharmacology & Therapeutics 200 (2019) 148–178 Contents lists available at ScienceDirect Pharmacology & Therapeutics journal homepage: www.elsevier.com/locate/pharmthera Biased signaling of G protein coupled receptors (GPCRs): Molecular determinants of GPCR/transducer selectivity and therapeutic potential Mohammad Seyedabadi a,b, Mohammad Hossein Ghahremani c, Paul R. Albert d,⁎ a Department of Pharmacology, School of Medicine, Bushehr University of Medical Sciences, Iran b Education Development Center, Bushehr University of Medical Sciences, Iran c Department of Toxicology–Pharmacology, School of Pharmacy, Tehran University of Medical Sciences, Iran d Ottawa Hospital Research Institute, Neuroscience, University of Ottawa, Canada article info abstract Available online 8 May 2019 G protein coupled receptors (GPCRs) convey signals across membranes via interaction with G proteins. Origi- nally, an individual GPCR was thought to signal through one G protein family, comprising cognate G proteins Keywords: that mediate canonical receptor signaling. However, several deviations from canonical signaling pathways for GPCR GPCRs have been described. It is now clear that GPCRs can engage with multiple G proteins and the line between Gprotein cognate and non-cognate signaling is increasingly blurred. Furthermore, GPCRs couple to non-G protein trans- β-arrestin ducers, including β-arrestins or other scaffold proteins, to initiate additional signaling cascades. Selectivity Biased Signaling Receptor/transducer selectivity is dictated by agonist-induced receptor conformations as well as by collateral fac- Therapeutic Potential tors. In particular, ligands stabilize distinct receptor conformations to preferentially activate certain pathways, designated ‘biased signaling’. In this regard, receptor sequence alignment and mutagenesis have helped to iden- tify key receptor domains for receptor/transducer specificity. -

Transcriptional and Post-Transcriptional Regulation of ATP-Binding Cassette Transporter Expression
Transcriptional and Post-transcriptional Regulation of ATP-binding Cassette Transporter Expression by Aparna Chhibber DISSERTATION Submitted in partial satisfaction of the requirements for the degree of DOCTOR OF PHILOSOPHY in Pharmaceutical Sciences and Pbarmacogenomies in the Copyright 2014 by Aparna Chhibber ii Acknowledgements First and foremost, I would like to thank my advisor, Dr. Deanna Kroetz. More than just a research advisor, Deanna has clearly made it a priority to guide her students to become better scientists, and I am grateful for the countless hours she has spent editing papers, developing presentations, discussing research, and so much more. I would not have made it this far without her support and guidance. My thesis committee has provided valuable advice through the years. Dr. Nadav Ahituv in particular has been a source of support from my first year in the graduate program as my academic advisor, qualifying exam committee chair, and finally thesis committee member. Dr. Kathy Giacomini graciously stepped in as a member of my thesis committee in my 3rd year, and Dr. Steven Brenner provided valuable input as thesis committee member in my 2nd year. My labmates over the past five years have been incredible colleagues and friends. Dr. Svetlana Markova first welcomed me into the lab and taught me numerous laboratory techniques, and has always been willing to act as a sounding board. Michael Martin has been my partner-in-crime in the lab from the beginning, and has made my days in lab fly by. Dr. Yingmei Lui has made the lab run smoothly, and has always been willing to jump in to help me at a moment’s notice.