DRUG NAME: Methotrexate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
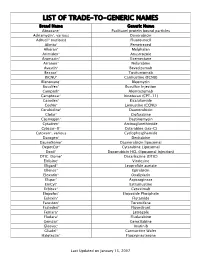
Trade-To-Generic Names
LIST OF TRADE-TO-GENERIC NAMES Brand Name Generic Name Abraxane® Paclitaxel protein bound particles Adriamycin®, various Doxorubicin Adrucil® (various) Fluorouracil Alimta® Pemetrexed Alkeran® Melphalan Arimidex® Anastrozole Aromasin® Exemestane Arranon® Nelarabine Avastin® Bevacizumab Bexxar® Tositumomab BiCNU® Carmustine (BCNU) Blenoxane® Bleomycin Busulfex® Busulfan Injection Campath® Alemtuzumab Camptosar® Irinotecan (CPT-11) Casodex® Bicalutamide CeeNu® Lomustine (CCNU) Cerubidine® Daunorubicin Clolar® Clofarabine Cosmegen® Dactinomycin Cytadren® Aminoglutethimide Cytosar-U® Cytarabine (ara-C) Cytoxan®, various Cyclophosphamide Dacogen® Decitabine DaunoXome® Daunorubicin liposomal DepotCyt® Cytarabine Liposomal Doxil® Doxorubicin HCL (liposomal injection) DTIC-Dome® Dacarbazine (DTIC) Eldisine® Vindesine Eligard® Leuprolide acetate Ellence® Epirubicin Eloxatin® Oxaliplatin Elspar® Asparaginase EmCyt® Estramustine Erbitux® Cetuximab Etopofos® Etoposide Phosphate Eulexin® Flutamide Fareston® Toremifene Faslodex® Fluvestrant Femara® Letrozole Fludara® Fludarabine Gemzar® Gemcitabine Gleevec® Imatinib Gliadel® Carmustine Wafer Halotestin® Fluoxymesterone Last Updated on January 15, 2007 Brand Name Generic Name Herceptin® Trastuzumab Hexalen® Altretamine Hycamtin® Topotecan Hydrea® Hydroxyurea Idamycin® Idarubicin Ifex® Ifosfamide Intron A® Interferon alfa-2b Iressa® Gefitinib Leukeran® Chlorambucil Leukine® Sargramostim Leustatin® Cladribine Lupron depot® Leuprolide acetate depot Lupron® Leuprolide acetate Matulane® Procarbazine Megace® -

PPE Requirements Hazardous Drug Handling
This document’s purpose is only to provide general guidance. It is not a definitive interpretation for how to comply with DOSH requirements. Consult the actual NIOSH hazardous drugs list and program regulations in entirety to understand all specific compliance requirements. Minimum PPE Required Minimum PPE Required Universal (Green) - handling and disposed of using normal precautions. PPE Requirements High (Red) - double gloves, gown, eye and face protection in Low (Yellow) - handle at all times with gloves and appropriate engineering Hazardous Drug Handling addition to any necessary controls. engineering controls. Moderate (Orange) -handle at all times with gloves, gown, eye and face protection (with splash potential) and appropirate engineering controls. Tablet Open Capsule Handling only - Contained Crush/Split No alteration Crush/Split Dispensed/Common Drug Name Other Drug Name Additional Information (Formulation) and (NIOSH CATEGORY #) Minimum PPE Minimum PPE Minimum PPE Minimum PPE Required required required required abacavir (susp) (2) ziagen/epzicom/trizivir Low abacavir (tablet) (2) ziagen/epzicom/trizivir Universal Low Moderate acitretin (capsule) (3) soriatane Universal Moderate anastrazole (tablet) (1) arimidex Low Moderate High android (capsule) (3) methyltestosterone Universal Moderate apomorphine (inj sq) (2) apomorphine Moderate arthotec/cytotec (tablet) (3) diclofenac/misoprostol Universal Low Moderate astagraf XL (capsule) (2) tacrolimus Universal do not open avordart (capsule) (3) dutasteride Universal Moderate azathioprine -

Handling of Hazardous Drugs Risk Prevention by Personal Protective Equipment Handling of Hazardous Drugs
HANDLING OF HAZARDOUS DRUGS RISK PREVENTION BY PERSONAL PROTECTIVE EQUIPMENT HANDLING OF HAZARDOUS DRUGS INTRODUCTION CONTENT The expression “antineoplastic drug” (ANPD) is often used synonymously together with “cytostatics” or Introduction 2 “chemotherapeutics”, however, these terms normally Definition of Risks 4 describe an overarching category to which other drug- classes belong. These types of drugs belong to drug- Causes for Risks 10 specialties summarized under the term “Hazardous Consequences 14 Drugs”, according to the CDC’s (Centers for Disease Control and Prevention) NIOSH4 alert in 2004. The Preventive Strategies 16 term ANPD describes in general the activity of these Risk Prevention 26 drugs against a neoplasm, characterizing an abnormal growth of tissue. In a recent systematic review and Literature 28 meta-analysis of the literature5 the expression “ANPD” Mandatory Information 31 is used in a general manner, therefore it is applied in this review, too. INTRODUCTION Antineoplastic drugs (ANPD) have been introduced ANPDs represent a broad and non-homogenous group for cancer treatment since the 1940s. More than of chemicals with a variety of structures, origins, ac- 12 million patients are treated with ANPDs each tivities and effects at the cellular level. They are cate- year. Nowadays the number of cancer diagnoses gorized according to their specific potential of toxicity is continuously increasing. or to their mechanisms of action, described in more detail in the chapter “Definition of risks”. Currently, the This brochure addresses the hazardous effects of anti- list encompassing ANPDs used in daily clinical practice neoplastic drugs, the importance of risk assessment contains more than 115 special drugs1. and standard precautions of personal protection as recommended by the 2004-NIOSH (National Institute During the 1970’s first concerns with respect to toxic for Occupational Safety and Health)-Alert and corre- side effects were raised further to cases that had been 1, 2, 3 sponding updates in 2010 / 2012 and 2016. -

Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications
International Journal of Molecular Sciences Review Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications Daniel Fernández-Villa 1, Maria Rosa Aguilar 1,2 and Luis Rojo 1,2,* 1 Instituto de Ciencia y Tecnología de Polímeros, Consejo Superior de Investigaciones Científicas, CSIC, 28006 Madrid, Spain; [email protected] (D.F.-V.); [email protected] (M.R.A.) 2 Consorcio Centro de Investigación Biomédica en Red de Bioingeniería, Biomateriales y Nanomedicina, 28029 Madrid, Spain * Correspondence: [email protected]; Tel.: +34-915-622-900 Received: 18 September 2019; Accepted: 7 October 2019; Published: 9 October 2019 Abstract: Bacterial, protozoan and other microbial infections share an accelerated metabolic rate. In order to ensure a proper functioning of cell replication and proteins and nucleic acids synthesis processes, folate metabolism rate is also increased in these cases. For this reason, folic acid antagonists have been used since their discovery to treat different kinds of microbial infections, taking advantage of this metabolic difference when compared with human cells. However, resistances to these compounds have emerged since then and only combined therapies are currently used in clinic. In addition, some of these compounds have been found to have an immunomodulatory behavior that allows clinicians using them as anti-inflammatory or immunosuppressive drugs. Therefore, the aim of this review is to provide an updated state-of-the-art on the use of antifolates as antibacterial and immunomodulating agents in the clinical setting, as well as to present their action mechanisms and currently investigated biomedical applications. Keywords: folic acid antagonists; antifolates; antibiotics; antibacterials; immunomodulation; sulfonamides; antimalarial 1. -

WHO Drug Information Vol
WHO Drug Information Vol. 31, No. 3, 2017 WHO Drug Information Contents Medicines regulation 420 Post-market monitoring EMA platform gains trade mark; Automated 387 Regulatory systems in India FDA field alert reports 421 GMP compliance Indian manufacturers to submit self- WHO prequalification certification 421 Collaboration 402 Prequalification process quality China Food and Drug Administration improvement initiatives: 2010–2016 joins ICH; U.S.-EU cooperation in inspections; IGDRP, IPRF initiatives to join 422 Medicines labels Safety news Improved labelling in Australia 423 Under discussion 409 Safety warnings 425 Approved Brimonidine gel ; Lactose-containing L-glutamine ; Betrixaban ; C1 esterase injectable methylprednisolone inhibitor (human) ; Meropenem and ; Amoxicillin; Azithromycin ; Fluconazole, vaborbactam ; Delafloxacin ; Glecaprevir fosfluconazole ; DAAs and warfarin and pibrentasvir ; Sofosbuvir, velpatasvir ; Bendamustine ; Nivolumab ; Nivolumab, and voxilaprevir ; Cladribine ; Daunorubicin pembrolizumab ; Atezolizumab ; Ibrutinib and cytarabine ; Gemtuzumab ozogamicin ; Daclizumab ; Loxoprofen topical ; Enasidenib ; Neratinib ; Tivozanib ; preparations ; Denosumab ; Gabapentin Guselkumab ; Benznidazole ; Ciclosporin ; Hydroxocobalamine antidote kit paediatric eye drops ; Lutetium oxodotreotide 414 Diagnostics Gene cell therapy Hightop HIV home testing kits Tisagenlecleucel 414 Known risks Biosimilars Warfarin ; Local corticosteroids Bevacizumab; Adalimumab ; Hydroquinone skin lighteners Early access 415 Review outcomes Idebenone -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Characterization and Separation of Platinum-Based Antineoplastic
separations Article Characterization and Separation of Platinum-Based Antineoplastic Drugs by Zwitterionic Hydrophilic Interaction Liquid Chromatography (HILIC)–Tandem Mass Spectrometry, and Its Application in Surface Wipe Sampling Stefano Dugheri 1,* , Nicola Mucci 2 , Enrico Mini 3, Donato Squillaci 2 , Giorgio Marrubini 4 , Gianluca Bartolucci 5 , Elisabetta Bucaletti 2, Giovanni Cappelli 2, Lucia Trevisani 2 and Giulio Arcangeli 2 1 Industrial Hygiene and Toxicology Laboratory, Occupational Medicine Unit, Careggi University Hospital, 50134 Florence, Italy 2 Department of Experimental and Clinical Medicine, University of Florence, 50134 Florence, Italy; nicola.mucci@unifi.it (N.M.); donato.squillaci@unifi.it (D.S.); elisabetta.bucaletti@unifi.it (E.B.); giovanni.cappelli@unifi.it (G.C.); lucia.trevisani@unifi.it (L.T.); giulio.arcangeli@unifi.it (G.A.) 3 Department of Health Sciences, University of Florence, 50134 Florence, Italy; enrico.mini@unifi.it 4 Department of Drug Sciences, University of Pavia, Via Taramelli 12, 27100 Pavia, Italy; [email protected] 5 Department of Neurosciences, Psychology, Drug Research and Child Health, University of Florence, 50019 Sesto Fiorentino, Italy; gianluca.bartolucci@unifi.it Citation: Dugheri, S.; Mucci, N.; * Correspondence: stefano.dugheri@unifi.it Mini, E.; Squillaci, D.; Marrubini, G.; Bartolucci, G.; Bucaletti, E.; Cappelli, Abstract: Platinum-based antineoplastic drugs (PtADs) are among the most important and used G.; Trevisani, L.; Arcangeli, G. families of chemotherapy drugs, which, -

BC Cancer Protocol Summary for Treatment of Lymphoma with Dose- Adjusted Etoposide, Doxorubicin, Vincristine, Cyclophosphamide
BC Cancer Protocol Summary for Treatment of Lymphoma with Dose- Adjusted Etoposide, DOXOrubicin, vinCRIStine, Cyclophosphamide, predniSONE and riTUXimab with Intrathecal Methotrexate Protocol Code LYEPOCHR Tumour Group Lymphoma Contact Physician Dr. Laurie Sehn Dr. Kerry Savage ELIGIBILITY: One of the following lymphomas: . Patients with an aggressive B-cell lymphoma and the presence of a dual translocation of MYC and BCL2 (i.e., double-hit lymphoma). Histologies may include DLBCL, transformed lymphoma, unclassifiable lymphoma, and intermediate grade lymphoma, not otherwise specified (NOS). Patients with Burkitt lymphoma, who are not candidates for CODOXM/IVACR (such as those over the age of 65 years, or with significant co-morbidities) . Primary mediastinal B-cell lymphoma Ensure patient has central line EXCLUSIONS: . Cardiac dysfunction that would preclude the use of an anthracycline. TESTS: . Baseline (required before first treatment): CBC and diff, platelets, BUN, creatinine, bilirubin. ALT, LDH, uric acid . Baseline (required, but results do not have to be available to proceed with first treatment): results must be checked before proceeding with cycle 2): HBsAg, HBcoreAb, . Baseline (optional, results do not have to be available to proceed with first treatment): HCAb, HIV . Day 1 of each cycle: CBC and diff, platelets, (and serum bilirubin if elevated at baseline; serum bilirubin does not need to be requested before each treatment, after it has returned to normal), urinalysis for microscopic hematuria (optional) . Days 2 and 5 of each cycle (or days of intrathecal treatment): CBC and diff, platelets, PTT, INR . For patients on cyclophosphamide doses greater than 2000 mg: Daily urine dipstick for blood starting on day cyclophosphamide is given. -

Weak Interaction of the Antimetabolite Drug Methotrexate with a Cavitand Derivative
International Journal of Molecular Sciences Article Weak Interaction of the Antimetabolite Drug Methotrexate with a Cavitand Derivative Zsolt Preisz 1,2, Zoltán Nagymihály 3,4, Beáta Lemli 1,4 ,László Kollár 3,4 and Sándor Kunsági-Máté 1,2,4,* 1 Institute of Organic and Medicinal Chemistry, Medical School, University of Pécs, Szigeti 12, H-7624 Pécs, Hungary; [email protected] (Z.P.); [email protected] (B.L.) 2 Department of General and Physical Chemistry, Faculty of Sciences, University of Pécs, Ifjúság 6, H 7624 Pécs, Hungary 3 Department of Inorganic Chemistry, Faculty of Sciences, University of Pécs, Ifjúság 6, H 7624 Pécs, Hungary; [email protected] (Z.N.); [email protected] (L.K.) 4 János Szentágothai Research Center, University of Pécs, Ifjúság 20, H-7624 Pécs, Hungary * Correspondence: [email protected]; Tel.: +36-72-503600 (ext. 35449) Received: 2 June 2020; Accepted: 16 June 2020; Published: 18 June 2020 Abstract: Formation of inclusion complexes involving a cavitand derivative (as host) and an antimetabolite drug, methotrexate (as guest) was investigated by photoluminescence measurements in dimethyl sulfoxide solvent. Molecular modeling performed in gas phase reflects that, due to the structural reasons, the cavitand can include the methotrexate in two forms: either by its opened structure with free androsta-4-en-3-one-17α-ethinyl arms or by the closed form when all the androsta-4-en-3-one-17α-ethinyl arms play role in the complex formation. Experiments reflect enthalpy driven complex formation in higher temperature range while at lower temperature the complexes are stabilized by the entropy gain. -

Public Assessment Report
Public Assessment Report Tifix 150mg and 500mg film-coated tablets Capecitabine BE/H/195/01-02/DC BE licence no: BE423552-BE423561 Applicant: Alfred E. Tiefenbacher, Germany Date: 27-04-2012 Toepassingsdatum : 15-09-10 Page 1 of 17 Blz. 1 van 17 This assessment report is published by the Federal Agency for Medicines and Health Products following Article 21 (3) and (4) of Directive 2001/83/EC, amended by Directive 2004/27/EC and Article 25 paragraph 4 of Directive 2001/82/EC as amended by 2004/28/EC. The report comments on the registration dossier that was submitted to the Federal Agency for Medicines and Health Products and its fellow organisations in all concerned EU member states. It reflects the scientific conclusion reached by the Federal Agency for Medicines and Health Products and all concerned member states at the end of the evaluation process and provides a summary of the grounds for approval of a marketing authorisation. This report is intended for all those involved with the safe and proper use of the medicinal product, i.e. healthcare professionals, patients and their family and carers. Some knowledge of medicines and diseases is expected of the latter category as the language in this report may be difficult for laymen to understand. This assessment report shall be updated by a following addendum whenever new information becomes available. To the best of the Federal Agency for Medicines and Health Products’ knowledge, this report does not contain any information that should not have been made available to the public. The Marketing Autorisation Holder has checked this report for the absence of any confidential information. -

ASHP Guidelines on Handling Hazardous Drugs
132 Drug Distribution and Control: Preparation and Handling–Guidelines ASHP Guidelines on Handling Hazardous Drugs ASHP published its first guidance on hazardous drugs (HDs) Because newer studies have shown that contamination in 1983 as part of the 1983–84 ASHP Practice Spotlight: Safe is widespread in healthcare settings and that more workers Handling of Cytotoxic Drugs.1,2 This was followed by tech- than previously thought are exposed, these guidelines should nical assistance bulletins in 1985 and 1990 and the ASHP be implemented wherever HDs are received, stored, pre- Guidelines on Handling Hazardous Drugs in 2006.3-5 The pared, transported, administered, or disposed.8-11 2006 guidelines were created to harmonize with the National Comprehensive reviews of the literature covering an- Institute for Occupational Safety and Health (NIOSH) Alert: ecdotal and case reports of surface contamination, worker ex- 6,9,12 Preventing Occupational Exposure to Antineoplastic and posure, and risk assessment are available from NIOSH, Other Hazardous Drugs in Health Care Settings issued in the Occupational Safety and Health Administration 13,14 15-20 2004.6 The ASHP 2006 HD guidelines were current to 2005. (OSHA), and individual authors. The primary goal In 2007, the United States Pharmacopeial Convention of this document is to provide recommendations for the safe revised United States Pharmacopeia (USP) chapter 797 handling of HDs. These guidelines represent the research (Pharmaceutical Compounding—Sterile Preparations)7 to and recommendations of many groups and individuals who harmonize with the NIOSH 2004 Alert. It became effective have worked tirelessly over decades to reduce the potential May 1, 2008, establishing many of the NIOSH recommenda harmful effects of HDs on healthcare workers. -

Targeted Therapies and Immunotherapy: General Principles (Oncology) – CE
Targeted Therapies and Immunotherapy: General Principles (Oncology) – CE ALERT Don appropriate personal protective equipment (PPE) based on the patient’s signs and symptoms and indications for isolation precautions. Refer to Oncology Nursing Society (ONS) interim guidelines for PPE recommendations during an emergent shortage of PPE (e.g., pandemic).13 Hypersensitivity reactions, such as anaphylaxis, may occur with targeted therapy and immunotherapy; therefore, frequent assessments and monitoring are required. Only qualified physicians, physician assistants, advanced practice registered nurses (APRNs), or nurses with demonstrated competency administer antineoplastic therapies. Refer to the professional’s regulatory scope of practice and the organization’s practice. Take steps to eliminate interruptions and distractions during medication preparation. OVERVIEW Normal cell reproduction, growth, and apoptosis are controlled by complex signaling pathways at the extracellular and intracellular levels. Malfunctioning of these pathways occurs in malignancies, leading to increased proliferation, tissue invasion, metastases, and apoptosis inhibition.18 Development of targeted therapies overcame the lack of selectivity associated with conventional antineoplastic therapy by targeting specific protein pathways.5 Although these pathways can be present in normal tissue, they are overexpressed or mutated in cancerous tissue.1 Cancer immunotherapy represents precision medicine, which helps the immune system fight cancer and offers a type of targeted or personalized therapy.4,11 In comparison, traditional cytotoxic chemotherapy attacks both malignant and nonmalignant cells, causing disruption of the cell cycle and other cell functions. Targeted therapy may not be more or less effective than traditional antineoplastic therapies, but it offers a unique approach to the treatment of cancer and other diseases and has toxicities different from those of traditional antineoplastic therapy.