Processes in Biological Vision: Electrochemistry of The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
A3. Neuron.Pdf
NEURON A3 (1) Neuron Last updated: April 20, 2019 NEURON .................................................................................................................................................... 1 IMMUNOHISTOCHEMICAL MARKERS FOR NEURONS ................................................................................ 2 CELL BODY (SOMA, PERIKARYON).......................................................................................................... 2 PROCESSES ............................................................................................................................................. 3 FUNCTIONAL NEURON CLASSIFICATION (BASIC PLAN OF NERVOUS SYSTEM) .......................................... 5 NEUROTROPHINS ..................................................................................................................................... 7 EXCITATION & CONDUCTION ................................................................................................................. 7 RESTING POTENTIAL .............................................................................................................................. 8 ACTION POTENTIAL ................................................................................................................................ 8 ELECTROTONIC POTENTIALS & LOCAL RESPONSE ............................................................................... 10 Local current flow ......................................................................................................................... -

Morphine and Opioid Peptides Reduce Inhibitory Synaptic Potentials in Hippocampal Pyramidal Cells in Vitro Without Alteration Of
Proc. Natl Acad. Sci. USA Vol. 78, No. 8, pp. 5235-5239, August 1981 Neurobiology Morphine and opioid peptides reduce inhibitory synaptic potentials in hippocampal pyramidal cells in vitro without alteration of membrane potential (opiates/intracellular recording/disinhibition/inhibitory postsynaptic potential/excitatory postsynaptic potential) G. R. SIGGINS* AND W. ZIEGLGANSBERGER Max Planck Institute for Psychiatry, Department of Neuropharmacology, Munich, Federal Republic of Germany Communicated by Dr. Floyd E. Bloom, April 24, 1981 ABSTRACT We used intracellular recording in the hippocam- and reduces evoked IPSPs, leading to paroxysmal depolariza- pal slice in vitro to characterize further the mechanisms behind tion shifts. the unusual excitatory action of opiates and opioid peptides on The reason for these discrepancies is unknown, although they hippocampal pyramidal cells in vivo. No significant effect on rest- may arise from the complicated feedback circuitry of the hip- ing membrane potential, input resistance, or action potential size pocampus and the close interrelationship between excitatory in cortical area 1 (CAI) pyramidal cells was observed with mor- and inhibitory mechanisms in the pyramidal cell. Thus, it is phine sulfate, ,f-endorphin, [Met5]enkephalin, or [D-Ala2, D- difficult to explain how opiates might elevate EPSP size in the Leu5]enkephalin at 1-50 ,LM. However, in all cells studied, these pyramidal cell without concomitant increases in IPSPs. agents markedly reduced the size of inhibitory postsynaptic po- To help tentials generated by stimulation of the stratum radiatum or al- clarify these discrepancies, we pursued ongoing studies of the veus. Excitatory postsynaptic potentials were also diminished in action ofopioids on both EPSPs and IPSPs in the same pyram- many of these cells. -

Structure and Measurement of the Brain Lecture Notes
Structure and Measurement of the brain lecture notes Marty Sereno 2009/2010 !"#$%&'(&#)*%$#&+,'-&.)"/*"&.*)*-'(0&1223 Neurons and Models Lecture 1 Topics • Membrane (Nernst) Potential • Action potential/Voltage-gated channels • Post-synaptic potentials, ligand gated channels • Dendritic propagation equivalent circuits • NMDA channels and synaptic plasticity • Spike timing dependent plasticity (STDP) How does the brain work? • 100 billion neurons in the human brain • 10 14 synapses (1000-5000 per neuron) from molecular level to systems level 8 10 Membrane Potential • Vm (membrane pot.) due to *resting* channels • = voltage difference across the membrane • I. different ions have different concentration gradients across the membrane • ion species: K+, Na+, Cl-, Ca++ • II. membrane is semi-permeable - most resting channels are K+ (leaky) channels 11 Membrane Potential (Vm) • ~ -70 mV (depends on cell type) • semi-permeable membrane: K+ • differential concentration gradients of K+, Na+, Cl-, Ca++ 12 Na+ - K+ pump • 3 Na+ out, 2 K+ in • moves ions against their concentration gradient • re-establishes concentration gradients 13 note: • voltage & concentration difference only immediately across membrane 14 Purpose of resting potential? • signaling is a brief deviation from the resting potential; • to signal information, must have a baseline/ resting state so incoming information isn’t drowned in noise 15 Nernst Potential • equilibrium potential for one ion • = reversal potential • when concentration gradient force balances out electrical force -

Passive Electrical Properties of the Neuron
Passive electrical properties of the neuron Ricardo C. Araneda [email protected]/4-4539 Fall 2004 This lecture take notes from the following books The following books were used for the lecture: “From neuron to brain” Nicholls et al., 3rd Ed. “Ion channels of excitable membranes” B. Hille, 3rd Ed. “Fundamental neuroscience” Zigmond et al. “Principles of neuroscience” Kandel et al., 4th Ed. Review of basic concepts Experiment 1 Cell membrane ◆Concentration Gradient NaCl NaCl 10 mM 100 mM in out Measure membrane potential ◆Concentration Gradient NaCl NaCl 10 mM 100 mM in out V= ?? Measure membrane potential ◆Concentration Gradient NaCl NaCl ◆ membrane is equally 10 mM 100 mM permeable in out to both Na and Cl V= ?? Membrane is selectively permeable to Sodium in out + Na + Na _ _ _ Cl Cl Cl Na + _ + Cl Concentration Gradient Na Na + _ Cl _ + Cl Na Na + Na + _ Cl _ _ Cl Cl Na + Membrane is selectively permeable to Sodium in out + Na + Na _ _ _ Cl Cl Cl Na + _ + Cl Concentration Gradient Na Na + _ Cl _ + Cl Na Na + Na + _ Cl _ _ Cl Cl Na + Membrane is selectively permeable to Sodium in out + Na + Na _ _ _ Cl Cl Cl Na + _ + Cl Concentration Gradient Na Cl Excess of Na+ - Excess of positive ions negative ions Potential difference _ + Cl Na Na + Na + _ Cl _ _ Cl Cl Na + Membrane potential ◆Concentration Gradient across the membrane ◆Membrane is selectively permeable to ions NaCl NaCl V = RT log [out] F [in] 10 mM 100 mM in out VNa = + 58 mV VCl = - 58 mV Membranes as capacitors Internal conducting External conducting solution solution (ions) (ions) Thin insulating layer (membrane, 4nm) Membranes as Resistors Internal conducting External conducting solution solution (ions) (ions) Ion channels Voltage-gated, NT- gated etc. -

The Axon to the Periphery. Physiologists of Prestige Deemed Iterroneous
592 PHYSIOLOGY: LORENTE DE N6 AND CONDOURIS PROC. N. A. S. zero and ceiling is an expression in the inverse sense of the rate at which the meta- bolic processes are operating. It is of interest that the dendritic membrane, given adequate oxygen, normal carbon dioxide and maintained at normal body temperature, should be set at a level of polarization so far from that at which maximal response is possible. One must suppose that dendritic conduction with decrement toward the tips is the normal occurrence and important to the normal working of the motoneurons. It would occur, for instance, not only in artificial "antidromic" action, but also in association with monosynaptic reflex transmission, for the presynaptic endings concerned are located on the cell body and the thick proximal dendrites,'1' 12 and the impulse generated there would travel toward the dendrite tips as well as along the axon to the periphery. 1 Renshaw, B., "Effects of presynaptic volleys on spread of impulses over the soma of the motoneuron," J. Neurophysiol., 5, 235-243 (1942). 2 Lorente de N6, R., "Action potential of the motoneurons of the hypoglossus nucleus," J. Cell. Comp. Physiol., 29, 207-288 (1947). 3 Lloyd, D. P. C., "The interaction of antidromic and orthodromic volleys in a segmental spinal motor nucleus," J. Neurophysiol., 6, 143-152 (1943). 4 Lloyd, D. P. C., "Electrical signs of impulse conduction in spinal motoneurons," J. Gen. Physiol., 35, 255-288 (1951). 6 Lloyd, D. P. C., "Note on the lateral dendrites of plantar motoneurons," these PROCEEDINGS, 45, 586-588 (1959). 6 Chang, H. -

The Effects of Postsynaptic Levels of Cyclic AMP on Excitatory and Inhibitory Responses of an Identified Central Neuron
The Journal of Neuroscience, March 1989, g(3): 784-797 The Effects of Postsynaptic Levels of Cyclic AMP on Excitatory and Inhibitory Responses of an Identified Central Neuron Laura R. Wolszon’ and Donald S. Faber2 ‘Department of Biophysical Sciences, and *Department of Physiology, State University of New York at Buffalo, Buffalo, New York 14214 The effects of CAMP on Mauthner (M-) cell excitatory and increaseboth the conductance of gap junctions and the number inhibitory responses were studied in viva. Cyclic AMP was of electrotonic junctions formed (seeSpray and Bennett, 1985) iontophoresed into the M-ceil lateral dendrite, after which the effects of CAMP on neuronal coupling have been studied in we monitored the changes in cellular responsiveness to only 2 preparations thus far. Specifically, (1) Kessleret al. (1984) stimulation of 2 classes of identified presynaptic cells: (1) found that CAMP increasesthe incidence of formation of gap excitatory fibers from the posterior branch of the ipsilateral junctions in cultured neonatal rat sympathetic neurons, and (2) eighth cranial nerve and (2) inhibitory interneurons activated elevated levels of CAMP have been found to decreasethe con- by the M-cell collateral and commissural networks. We found ductance through electrotonic junctions in retinal horizontal that postsynaptic injections of CAMP increased the magni- cells (Piccolino et al., 1982; Teranishi et al., 1982; Lasater and tudes of the electrically and chemically mediated EPSPs Dowling, 1985). Similarly, information relating to the modifi- from the eighth nerve and enhanced M-cell inhibitory re- cation of inhibitory synapsesis also somewhatlimited; while a sponses as well. Furthermore, CAMP augmented paired-pulse great deal is now known about modulatory mechanismsregu- facilitation of both types of excitatory potentials. -
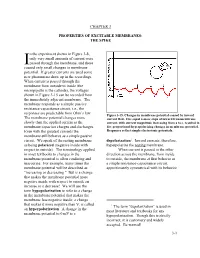
THE SPIKE in the Experiment Shown in Figure 3-8, Only Very Small Amounts Of
CHAPTER 3 PROPERTIES OF EXCITABLE MEMBRANES: THE SPIKE n the experiment shown in Figure 3-8, only very small amounts of current were Ipassed through the membrane, and these caused only small changes in membrane potential. If greater currents are used some new phenomena show up in the recordings. When current is passed through the membrane from outside to inside (the micropipette is the cathode), the voltages shown in Figure 3-15 can be recorded from the immediately adjacent membrane. The membrane responds as a simple passive resistance-capacitance circuit, i.e., the responses are predictable from Ohm’s law. Figure 3-15. Changes in membrane potential caused by inward The membrane potential changes more current flow. Five equal 4-msec steps of inward transmembrane slowly than the applied current as the current, with current magnitude increasing from a to e, resulted in membrane capacitor charges and discharges. five proportional hyperpolarizing changes in membrane potential. Even with the greatest currents the Responses reflect simple electrotonic potentials. membrane still behaves as a simple passive circuit. We speak of the resting membrane depolarization1. Inward currents, therefore, as being polarized (negative inside with hyperpolarize the resting membrane. respect to outside). The terminology applied When current is passed in the other in most textbooks to changes in the direction across the membrane, from inside membrane potential is often confusing and to outside, the membrane at first behaves as inaccurate. For example, many times the a simple resistance-capacitance circuit, membrane potential will be described as approximately symmetrical with its behavior "increasing or decreasing." But is a change that makes the membrane potential more negative inside with respect to outside an increase or a decrease? We will use the term hypopolarization to refer to a change in the membrane potential that makes the membrane less negative inside; a change that makes it more negative than V is called r 1 The term “depolarization” is used in an hyperpolarization. -

Core Conductor Theory and Cable Properties of Neurons. In
CHAPTER 3 Core conductor theory and cable properties of neurons Mathematical Research Branch, National Institute of Arthritis, Metabolism, I I A and Digestive Diseuses, National Institutes of Health, Bethesda, Maryland CHAPTER CONTENTS Ohm's law for core current Conservation of current Introduction Relation of membrane current to V, Core conductor concept Effect of assuming extracellular isopotentiality Perspective Passive membrane model Comment Resulting cable equation for simple case Reviews and monographs Physical meaning of cable equation terms Brief Historical Notes Physical meaning of T Early electrophysiology Physical meaning of A Electrotonus Electrotonic distance, length, and decrement Passive membrane electrotonus Effect of placing axon in oil Passive versus active membrane Effect of applied current Cable theory Comment on sign conventions Core conductor concept Effect of synaptic membrane conductance Core conductor theory Effect of active membrane properties Estimation of membrane capacitance Input Resistance and Steady Decrement with Distance Resting membrane resistivity Note on correspondence with experiment Passive cable parameters of invertebrate axons Cable of semi-infinite length Importance of single axon preparations Comments about R,, G,, core current, and input current Estimation of parameters for myelinated axons Doubly infinite length Space and voltage clamp Case of voltage clamps at XI and Xp Dendritic Aspects of' Neurons Relations between axon parameters Axon-dendrite contrast Finite length: effect of boundary -

Protein Kinase C Enhances Electrical Synaptic Transmission by Acting on Junctional and Postsynaptic Ca2+ Currents Christopher C
This Accepted Manuscript has not been copyedited and formatted. The final version may differ from this version. Research Articles: Cellular/Molecular Protein kinase C enhances electrical synaptic transmission by acting on junctional and postsynaptic Ca2+ currents Christopher C. Beekharry, Yueling Gu and Neil S. Magoski Department of Biomedical and Molecular Sciences, Experimental Medicine Graduate Program, Queen's University, Kingston, ON, K7L 3N6, Canada DOI: 10.1523/JNEUROSCI.2619-17.2018 Received: 12 September 2017 Revised: 15 January 2018 Accepted: 2 February 2018 Published: 13 February 2018 Author contributions: NSM designed research; CBB and AG performed research; CBB, AG, and NSM analyzed data; CBB and NSM wrote the paper.; Supported by a Natural Sciences and Engineer Research Council discovery grant (NSM).; The authors thank CA London and HM Hodgson for technical assistance, as well as NM Magoski for critical review of the paper. Conflict of Interest: The authors declare no competing financial interests. Correspondence: Dr. N.S. Magoski, Department of Biomedical and Molecular Sciences, Queen's University, 4th Floor, Botterell Hall, 18 Stuart Street, Kingston, ON, K7L 3N6, Canada, Tel: (613) 533-3173, Fax: (613) 533-6880, E-mail: [email protected] Cite as: J. Neurosci ; 10.1523/JNEUROSCI.2619-17.2018 Alerts: Sign up at www.jneurosci.org/cgi/alerts to receive customized email alerts when the fully formatted version of this article is published. Accepted manuscripts are peer-reviewed but have not been through the copyediting, formatting, or proofreading process. Copyright © 2018 the authors 1 Protein kinase C enhances electrical synaptic transmission by 2+ 2 acting on junctional and postsynaptic Ca currents 3 4 5 by: 6 7 8 Christopher C. -

Response Due to Stimulation of Nerve Fiber When a Nerve Fiber Is Stimulated, Based on the Strength of Stimulus, Two Types of Response Develop: 1
UNIT-2: ELECTRICAL POTENTIALS OF NEURILEMMA 2020 Excitability is defined as the physiochemical change that occurs in a tissue when stimulus is applied. Nerve fibers have a low threshold for excitation than the other cells. Response Due to Stimulation of Nerve Fiber When a nerve fiber is stimulated, based on the strength of stimulus, two types of response develop: 1. Action potential or nerve impulse: Action potential develops in a nerve fiber when it is stimulated by a stimulus with adequate strength. Adequate strength of stimulus, necessary for producing the action potential in a nerve fiber is known as threshold or minimal stimulus. Action potential is propagated. 2. Electrotonic potential or local potential: When the stimulus with subliminal strength is applied, only electrotonic potential develops and the action potential does not develop. Electrotonic potential is nonpropagated. A. RESTING MEMBRANE POTENTIAL Resting membrane potential is defined as the electrical potential difference (voltage) across the cell membrane (between inside and outside of the cell) under resting condition. It is also called membrane potential, transmembrane potential, transmembrane potential difference or transmembrane potential gradient. When two electrodes are connected to a cathode ray oscilloscope through a suitable amplifier and placed over the surface of the muscle fiber, there is no potential difference, i.e. there is zero potential difference. But, if one of the electrodes is inserted into the interior of muscle fiber, potential difference is observed across the sarcolemma (cell membrane). There is negativity inside and positivity outside the muscle fiber. This potential difference is constant and is called resting membrane potential. The condition of the muscle during resting membrane potential is called polarized state. -

The Journal of General Physiology 138 PROLONGED ACTION POTENTIALS
PROLONGED ACTION POTENTIALS FROM SINGLE NODES OF RANVIER* B~ PAUL MUELLER$ (From The Rockefeller Institute) (Received for publication, January 20, 1958) Downloaded from http://rupress.org/jgp/article-pdf/42/1/137/1241752/137.pdf by guest on 25 September 2021 ABSTRACT The duration of action potentials from single nodes of Ranvier can be increased by several methods. Extraction of water from the node (e.g. by 2 to 3 M glycerin) causes increased durations up to 1000 msec. 1 to 5 rain. after application of the glycerin the duration of the action potential again decreases to the normal value. Another type of prolonged action potential can be observed in solutions which contain K or Rb ions at concentrations between 50 mM and 2 ~. The nodes respond only if the resting potential is restored by anodal current. The kinetics of these action potentials is slightly different. Their maximal durations are longer (up to 10 sec.). Like the normal action potential, they are initiated by cathodal make or anodal break. They also occur in external solutions which contain no sodium. The same type of action potentials as in KCI is found when the node is depolarized for some time (15 to 90 sec., 100 to 200 mv.) and is then stimulated by cathodal current. These action potentials require no K or Na ions in the external medium. Their maximal duration increases with the strength and duration of the preceding depolarization. The possible origin of the action potentials in KCI and after depolari- zation, and their relation to the normal action potentials and the negative after- potential are discussed. -

Electrotonic Interaction Between Muscle Fibers in the Rabbit Ventricle
Electrotonic Interaction between Muscle Fibers in the Rabbit Ventricle J. TILLE From the Department of Physiology, Monash University, Clayton, Victoria, Australia ABSTRACT Transmembrane potentials were recorded simultaneously from pairs of ventricular fibers in an isolated, regularly beating preparation. A double-barrelled microelectrode was used to record the potentials from, and to polarize, one fiber. A single microelectrode was used to record from a distant fiber. The existence of two systems of fibers, termed P and V, was confirmed. Histological evidence for the existence of two types of fibers is also presented. Electrotonic current spread was observed within both systems, electrotonic in- teraction between the two systems was rare and always weak. In the case of those pairs of fibers showing electrotonic interaction, the distance for an e-fold decrease in magnitude of the electrotonic potentials was found to be from 300 to 600 in P fibers and from 100 to 300 , in V fibers. However, no elec- trotonic interaction could be observed in the majority of V fiber pairs. More- over, the magnitude of the electrotonic potential did not decay monotonically with distance in any one direction. It is concluded that the rabbit ventricle cannot be regarded as a single freely interconnected syncytium. INTRODUCTION Using fibers in the rabbit right ventricle, Johnson and Tille (1960, 1961 b) found that voltage-current relationships, obtained by passing hyperpolarizing current during the repolarization phase of the action potential, were linear and that no regenerative repolarization could be elicited. They concluded that the membrane conductance of ventricular fibers is independent of the mem- brane potential during the repolarization phase of the action potential.