Unlted States Patent [19] [11] Patent Number: 4,469,452 Sharpless Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent 19 11) 4,045,383 Koff 45) Aug
United States Patent 19 11) 4,045,383 Koff 45) Aug. 30, 1977 (54) STABLE EMULSIONS AND IMPROVED (56) References Cited TEMPERATURE MONITORING FILMS FOREIGN PATENT DOCUMENTS PREPARED THEREFROM 1,138,590 1/1969 United Kingdom (75) Inventor: Arnold Koff, West Orange, N.J. OTHER PUBLICATIONS (73) Assignee: Hoffmann-La Roche Inc., Nutley, Chem. Abstract 48:225 h. N.J. Chem. Abstract 64:17,361 g. 21 Appl. No.: 651,010 Primary Examiner-Theodore Morris Attorney, Agent, or Firm-Samuel L. Welt; Bernard S. 22) Filed: Jan. 21, 1976 Leon; William M. Farley 57 ABSTRACT Related U.S. Application Data Improved temperature monitoring films formed from (63) Continuation of Ser. No. 221,408, Jan. 27, 1972, stable emulsions containing compounds capable of ex abandoned, which is a continuation-in-part of Ser. No. isting in the cholesteric liquid crystalline phase which 885,353, Dec. 15, 1969, abandoned. are protected and stabilized against aging, and the atmo 51 Int. C.’................................................ C09K 3/34 sphere by the inclusion of an antioxidant are provided. (52) U.S. C. ................................... 260/8; 23/230 LC; The thus-formed film materials are used in thermogra 106/135; 252/299 phy and/or thermometry. 58) Field of Search ........................ 106/135; 252/299; 260/8; 428/1; 23/230 LC 3 Claims, No Drawings 4,045,383 1. 2 viewed, has an apparent color which is a complement of STABLE EMULSONS AND MPROVED the color of the light transmitted through the film. TEMPERATURE MONTORING FILMS The terms "light” and "color" as used herein have a PREPARED THEREFROM broad connotation of referring to electromagnetic radi ation generally, rather than to solely visible radiation. -
United States Patent (19) ESSSÉÉ
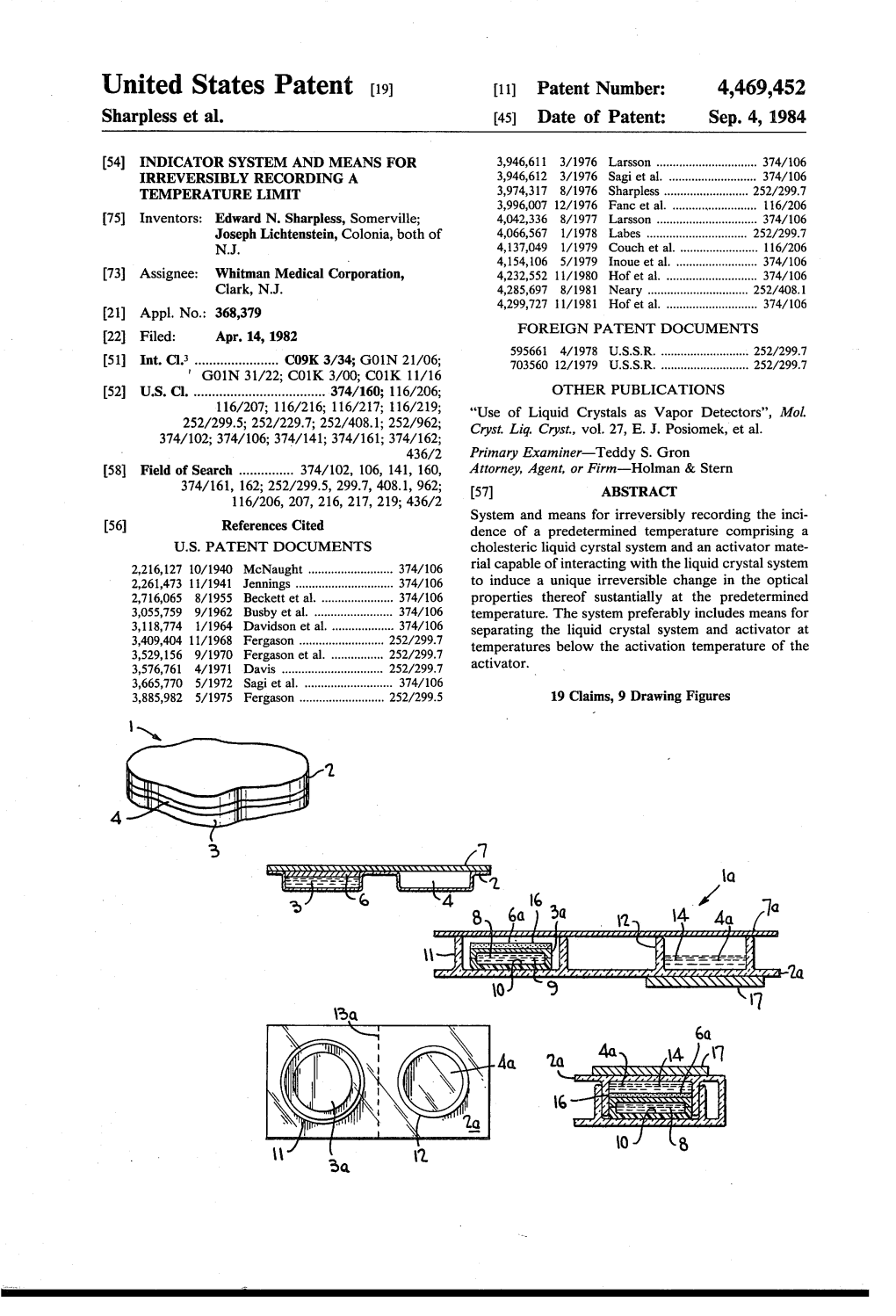
United States Patent (19) 11 Patent Number: 4,469,452 Sharpless et al. 45) Date of Patent: Sep. 4, 1984 54 NDCATOR SYSTEMAND MEANS FOR 3,946,611 3/1976 Larsson ............................... 374/106 RREVERSIBLY RECORDING A 3,946,612 3/1976 Sagi et al. ........................... 374/106 TEMPERATURE LIMIT 3,974,317 8/1976 Sharpless ... ... 252/299.7 3,996,007 12/1976 Fanc et al. .......................... 6/206 (75) Inventors: Edward N. Sharpless, Somerville; 4,042,336 8/1977 Larsson ............................... 374/106 Joseph Lichtenstein, Colonia, both of 4,066,567 1/978 Labes .......... ... 252/299.7 4,137,049 1/1979 Couch et al. ........................ 116/206 4,154,106 5/1979 Inoue et al. ......................... 374/106 73) Assignee: Whitman Medical Corporation, 4,232,552 11/1980 Hof et al. ............................ 374/106 Clark, N.J. 4,285,697 8/1981 Neary .......... ... 252A408.1 (21) Appl. No.: 368,379 4,299,727 1/1981 Hof et al. ............................ 374/106 22 Filed: Apr. 14, 1982 FOREIGN PATENT DOCUMENTS 59566 4/1978 U.S.S.R. ........................... 252/299.7 51 Int. Cl. ....................... C09K 3/34; G01N 21/06; 703560 12A1979 U.S.S.R. ........................... 252/299.7 31/22; CO1K 3/00; C01K 11/16 52 U.S. C. .................................... 374/160; 116/206; OTHER PUBLICATIONS 116/207; 116/216; 116/217; 116/219; "Use of Liquid Crystals as Vapor Detectors', Mol. 252/299.5; 252/229.7; 252/408.1; 252/962; Cryst. Liq. Cryst., vol. 27, E. J. Posiomek, et al. 374/102; 374/106,374/141; 374/161; 374/162; 436/2 Primary Examiner-Teddy S. -

Bioresorbable Microdroplet Lasers As Injectable Systems for Transient Thermal Sensing and Modulation
Article www.acsnano.org Bioresorbable Microdroplet Lasers as Injectable Systems for Transient Thermal Sensing and Modulation Daniel Franklin, Tyler Ueltschi, Andrea Carlini, Shenglian Yao, Jonathan Reeder, Benjamin Richards, Richard P. Van Duyne, and John A. Rogers* Cite This: ACS Nano 2021, 15, 2327−2339 Read Online ACCESS Metrics & More Article Recommendations *sı Supporting Information ABSTRACT: Minimally invasive methods for temperature sensing and thermal modulation in living tissues have extensive applications in biological research and clinical care. As alternatives to bioelectronic devices for this purpose, functional nanomaterials that self-assemble into optically active microstructures offer important features in remote sensing, injectability, and compact size. This paper introduces a transient, or bioresorbable, system based on injectable slurries of well-defined microparticles that serve as photopumped lasers with temperature-sensitive emission wave- lengths (>4−300 nm °C−1). The resulting platforms can act as tissue-embedded thermal sensors and, simultaneously, as distrib- uted vehicles for thermal modulation. Each particle consists of a spherical resonator formed by self-organized cholesteric liquid crystal molecules doped with fluorophores as gain media, encapsulated in thin shells of soft hydrogels that offer adjustable rates of bioresorption through chemical modification. Detailed studies highlight fundamental aspects of these systems including particle sensitivity, lasing threshold, and size. Additional experiments explore functionality as photothermal agents with active temperature feedback (ΔT =1°C) and potential routes in remote evaluation of thermal transport properties. Cytotoxicity evaluations support their biocompatibility, and ex vivo demonstrations in Casper fish illustrate their ability to measure temperature within biological tissues with resolution of 0.01 °C. This collective set of results demonstrates a range of multifunctional capabilities in thermal sensing and modulation. -

Synthesis of Cholesteryl Ester Liquid Crystals
Preparation of Cholesteryl Ester Liquid Crystals © 2011 by David A. Katz. All rights reserved The procedure for temperature sensitive liquid crystals is based on G. H. Brown and J. J. Wolken, Liquid Crystals and Biological Systems, Academic Press, NY, 1979, pp. 165-167 and W. Elser and R. D. Ennulat, Adv. Liq. Cryst. 2, 73 (1976). This experiment is modified from the Lab Manual for Nanoscale Science and Technology at http://mrsec.wisc.edu/Edetc/nanolab/LC_prep/index.html The procedure for pressure sensitive liquid crystals is based on Griffiths, Jonathan S., Experimental Chemistry, Stockton State College, Pomona, NJ, 1988 Additional information and procedures added by David A. Katz, Department of Chemistry, Pima Community College Liquid crystals are organic compounds that are in a state between liquid and solid forms. They are viscous, jelly-like materials that resemble liquids in certain respects (viscosity) and crystals in other properties (light scattering and reflection). Liquid crystals must be geometrically highly anisotropic (having different optical properties in different directions)-usually long and narrow - and revert to an isotropic liquid (same optical properties in all directions) through thermal action (heat) or by the influence of a solvent. Liquid crystals are classified as: smectic: molecules arranged in horizontal layers or strata and are standing on end either vertically or at a tilt. nematic: molecules possess a high degree of long-range order with their long axes approximately parallel, but without the distinct layers of the smectic crystals. lyotropic: molecules consist of a nonpolar hydrocarbon chain with a polar head group. In a solvent, such as water, the water molecules are sandwiched between the polar heads of adjacent layers while the hydrocarbon tails lie in a nonpolar environment. -

EXPLORATORY INVESTIGATION on the MEASUREMENT of SKIN FRICTION by MEANS of LIQUID CRYSTALS by Enrique J
NASA TECHNICAL NA SA TM X-17 74 MEMORANDUM ......v .-...... I >< c EXPLORATORY INVESTIGATION ON THE MEASUREMENT OF SKIN FRICTION BY MEANS OF LIQUID CRYSTALS by Enrique J. Klein and Angelo P. Margozzi Ames Research Center Moffett Field, Calif. NATIONAL AERONAUTICS AND SPACE ADMINISTRATION • WASHING TON, D. C. • MAY 1969 NASA TM X-1774 EXPLORA TORY INVESTIGATION ON THE MEASUREMENT OF SKIN FRICTION BY MEANS OF LIQUID CRYSTALS By Enrique J. Klein and Angelo P. Margozzi Ames Research Center Moffett Field, Calif. NATIONAL AERONAUTICS AND SPACE ADMINISTRATION For sale by the Clearinghouse for Federal Scientific and Technical Information Springfield, Vi rginia 22151 - CFSTI price $3.00 EXPLORATORY INVESTIGATION ON THE MEASUREMENT OF SKIN FRICTION BY MEANS OF LIQUID CRYSTALS 1 By Enrique J. Klein and Angelo P. Margozzi Ames Research Center SUMMARY A new method is proposed for directly measuring local skin friction in aerodynamic testing. It makes use of thin coatings of cholesteric liquid crystal mixtures that respond to shearing forces by changes in the wavelength of maximum light scattering. A calibration of shear versus wavelength was obtained for a sample substance; a static calibration was made to measure the effects of temperature, pressure, and angle of viewing; and the behavior of the substance was observed in a pipe-flow experiment. Preliminary results showed that the method appears feasible. However, for such a method to become widely applicable in wind-tunnel testing, further research will be needed in (1) obtaining liquid crystal mixtures sensitive over appropriate ranges on the shear scale, (2) preventing the occurrence of roughness on the surface of liquid crystal coatings subjected to aerodynamic shear forces, and (3) developing a suitable optical measuring technique for data acquisition. -

Sigma Biochemical Condensed Phase
Sigma Biochemical Condensed Phase Library Listing – 10,411 spectra This library provides a comprehensive spectral collection of the most common chemicals found in the Sigma Biochemicals and Reagents catalog. It includes an extensive combination of spectra of interest to the biochemical field. The Sigma Biochemical Condensed Phase Library contains 10,411 spectra acquired by Sigma-Aldrich Co. which were examined and processed at Thermo Fisher Scientific. These spectra represent a wide range of chemical classes of particular interest to those engaged in biochemical research or QC. The spectra include compound name, molecular formula, CAS (Chemical Abstract Service) registry number, and Sigma catalog number. Sigma Biochemical Condensed Phase Index Compound Name Index Compound Name 8951 (+)-1,2-O-Isopropylidene-sn-glycerol 4674 (+/-)-Epinephrine methyl ether .HCl 7703 (+)-10-Camphorsulfonic acid 8718 (+/-)-Homocitric acid lactone 10051 (+)-2,2,2-Trifluoro-1-(9-anthryl)ethanol 4739 (+/-)-Isoproterenol .HCl 8016 (+)-2,3-Dibenzoyl-D-tartaric acid 4738 (+/-)-Isoproterenol, hemisulfate salt 8948 (+)-2,3-O-Isopropylidene-2,3- 5031 (+/-)-Methadone .HCl dihydroxy-1,4-bis- 9267 (+/-)-Methylsuccinic acid (diphenylphosphino)but 9297 (+/-)-Miconazole, nitrate salt 6164 (+)-2-Octanol 9361 (+/-)-Nipecotic acid 9110 (+)-6-Methoxy-a-methyl-2- 9618 (+/-)-Phenylpropanolamine .HCl naphthaleneacetic acid 4923 (+/-)-Sulfinpyrazone 7271 (+)-Amethopterin 10404 (+/-)-Taxifolin 4368 (+)-Bicuculline 4469 (+/-)-Tetrahydropapaveroline .HBr 7697 (+)-Camphor 4992 (+/-)-Verapamil, -

United States Patent 19 11, 3,720,623 Cartmell Et Al
United States Patent 19 11, 3,720,623 Cartmell et al. (45 March 13, 1973 (54) ENCAPSULATED LIQUID CRYSTALS 3,499,112 3f 1970 Heilmeier et al............. 231230 LC X 75) Inventors: James W. Cartmell; Donald 3,578,844 5/1971 Churchill et al.................. 252/316X Churchill, both of Dayton, Ohio 3,600,060 8/1971 Churchill et al.................. 252/316X 73) Assignee: The National Cash Register Com Primary Examiner-Richard D. Lovering pany, Dayton, Ohio Attorney-E. Frank McKinney and Patrick P. Pacella 22 Filed: Feb. 8, 1971 (21) Appl. No.: 113,716 57 ABSTRACT A mixture of cholesteric liquid crystal material such as 52 U.S. Cl.............. 252/316, 231230 LC, 1 17137 R, cholesteryl nonanoate and nematic liquid crystal 1 17/100 A, 1171159, 2521403, 252/.408, material such as methoxybenzilidene-p-n-butylaniline 264/4, 350/160 P, 350/160 R is disclosed. The mixture can be encapsulated and em 51) Int. Cl..............................................Bo1j 13/02 ployed in temperature-sensitive visual display devices. 58) Field of Search ............... 252/316; 1171 100 A; The addition of nematic liquid crystal material to 231230 LC; 350/160 R, 160 P: 26414 cholesteric liquid crystal material enhances the brightness of films employing cholesteric liquid crystal 56) References Cited material and prolongs the usable life of the films. UNITED STATES PATENTS 6 Claims, No Drawings 3,341,466 9/1967 Brynko et al......................... 252/316 3,720,623 1. 2 ENCAPSULATED LIQUID CRYSTALS the usable lifetime. Also, by the addition of nematic liquid crystals, oleyl cholesteryl carbonate can be This invention relates to unencapsulated and encap eliminated from the mixtures, if desired. -

Cholesteric Liquid Crystals: Optics, Electro-Optics, and Photo-Optics
6 Cholesteric Liquid Crystals: Optics, Electro-optics, and Photo-optics Guram Chilaya Cholesteric liquid crystals (CLCs) show very distinctly that molecular struc- ture and external ®elds have a profound e¨ect on cooperative behavior and phase structure (see also Chapters 2 and 3). CLCs possess a supermolecular periodic helical structure due to the chirality of molecules. The spatial peri- odicity (helical pitch) of cholesterics can be of the same order of magnitude as the wavelength of visible light. If so, a visible Bragg re¯ection occurs. On the other hand, the helix pitch is very sensitive to the in¯uence of external conditions. A combination of these properties leads to the unique optical properties of cholesterics which are of both scienti®c and practical interest. The in¯uence of electric ®elds and light irradiation on the optical proper- ties of cholesteric structures are reviewed in this chapter. In Section 6.1 we consider the general optical properties of CLCs, such as Bragg di¨raction due to the periodical structure, refractive indices, and induced circular di- chroism. In the subsequent sections, electro-optic e¨ects and light-induced e¨ects are described. Several types of electro-optic e¨ects have been observed in cholesteric liquid crystals [1]±[3]. Section 6.2 of this chapter is focused on some of the most recent results, e.g., bistability, color change e¨ects, ``amorphous cholesterics,'' and the ¯exoelectric e¨ect. In addition, dielectric, hydrodynamic, and ¯exoelectric instabilities, as well as domain structures in cholesterics, are discussed brie¯y. Light-induced molecular reorientations and optical nonlinearities in transparent (nonabsorbing) and absorbing cho- lesterics, photostimulated shift of the pitch, optical bistability, and optical switching are presented in Section 6.3. -

Nov. 5, 1968 J. L. FERGASON 3,409,404 ANALYTICAL METHODS and DEVICES EMPLOYING CHOLESTERIC LIQUID CRYSTALLINE MATERIALS Filed Nov
Nov. 5, 1968 J. L. FERGASON 3,409,404 ANALYTICAL METHODS AND DEVICES EMPLOYING CHOLESTERIC LIQUID CRYSTALLINE MATERIALS Filed Nov. 13, 1963 2 Sheets-Sheet 1 4/70 M j 20 2/ 26 22 INVENTOR. JAMES L . FEQGASO/V A 77'ORNEY. 1 3,409,404 United States Patent 0 1C@ Patented Nov. 5, 1968 1 2 It is optically negative, while smectic and nematic struc ' ' 3,409,404 ' ANALYTICAL METHODS AND DEVICES EM tures are optically positive. (2) The structure is opti PLOYING 'CHOLESTERIC LIQUID CRYSTAL cally active. It shows strong optical rotatory power. (3) LINE MATERIALS When illuminated with white light, the most striking James , L. Fergason, Verona, Pa., assignor to Westing property of the cholesteric structure is that it scatters house Electric Corporation, Pittsburgh, Pa., a corpora light selectively to ‘give vivid colors. A cholesteric mate tion of Pennsylvania ‘ rial exhibits a scattering peak having a bandwidth of Filed Nov. 13, 1963, Ser. No. 323,341 about 200 angstroms that occurs in or between the infra 19 ‘Claims. (Cl. 23-230) red and ultraviolet portions of the spectrum. (4) In the 10 cholesteric structure, one circular polar component of the incident beam is completely unaffected. For the dextro ABSTRACT OF THE DISCLOSURE cholesteric structure, it is only the circular. polarized The optical properties of a chloresteric liquid crystal beam with counter-clockwise rotating electric vector line material are changed when the cholesteric material which is re?ected. (The sign of ‘rotation refers to an ob is contacted with another material. A variety of mate 15 server who looks in the direction of the incident light.) rials, particularly vapors, are identi?ed by observing their Levo cholesteric structures have the reverse effect. -

Nanotechnology Experiments for General Chemistry Laboratory Classes
NANOTECHNOLOGY EXPERIMENTS FOR GENERAL CHEMISTRY LABORATORY CLASSES David A. Katz Chemist, educator, and consultant Tucson, Arizona, U.S.A. Email: [email protected] Web site: http://www.chymist.com • Nanotechnology – Major area of research and development – Only now being introduced into textbooks for general chemistry – Almost no inclusion in the student laboratory. • Lab procedures and kits developed at the Materials Research Science and Engineering Center (MRSEC) at the University of Wisconsin‐ Madison http://mrsec.wisc.edu/Edetc/index.html (Go to video lab manual) Courses CHM 121IN, Chemistry and Society CHM 125IN, Consumer Chemistry Non‐major courses Taught as a hands‐on learning courses Experiments introduced in 2003 CHM 151‐152IN, General Chemistry ENG 110IN, Solid State Chemistry LED and solar cell experiments included in laboratory Mood Rings Dark blue: Happy, romantic or passionate Blue: Calm or relaxed Blue-green: Somewhat relaxed Green: Normal or average Amber: A little nervous or anxious Gray: Very nervous or anxious Black: Stressed, tense or feeling harried Liquid Crystals Both pressure sensitive and temperature sensitive (thermochromic) mixtures are prepared Liquid Crystals • Organic compounds in a state between liquid and solid • Viscous, jelly‐like materials that resemble liquids in viscosity and crystals in lightscattering and reflection • Highly anisotropic (having different optical properties in different directions) ‐ usually long and narrow ‐ and revert to an isotropic liquid (same optical properties in all directions) through thermal action (heat) or by the influence of a solvent. Cholesterol Cholesteryl Ester Liquid Crystals H H H H H H HO Cl OO cholesterol cholesteryl chloride cholesteryl benzoate O OO OO cholesteryl pelargonate cholesteryl oleyl carbonate Types of Cholesteryl Liquid Crystals Lyotropic Molecules consist of a nonpolar hydrocarbon chain with a polar head group. -

Sigma Biochemical Condensed Phase
Sigma Biochemical Condensed Phase Library Listing – 10,411 spectra This library provides a comprehensive spectral collection of the most common chemicals found in the Sigma Biochemicals and Reagents catalog. It includes an extensive combination of spectra of interest to the biochemical field. The Sigma Biochemical Condensed Phase Library contains 10,411 spectra acquired by Sigma-Aldrich Co. which were examined and processed at Thermo Fisher Scientific. These spectra represent a wide range of chemical classes of particular interest to those engaged in biochemical research or QC. The spectra include compound name, molecular formula, CAS (Chemical Abstract Service) registry number, and Sigma catalog number. Sigma Biochemical Condensed Phase Index Compound Name Index Compound Name 8951 (+)-1,2-O-Isopropylidene-sn-glycerol 8718 (+/-)-Homocitric acid lactone 7703 (+)-10-Camphorsulfonic acid 4739 (+/-)-Isoproterenol .HCl 10051 (+)-2,2,2-Trifluoro-1-(9-anthryl)ethanol 4738 (+/-)-Isoproterenol, hemisulfate salt 8016 (+)-2,3-Dibenzoyl-D-tartaric acid 5031 (+/-)-Methadone .HCl 8948 (+)-2,3-O-Isopropylidene-2,3- 9267 (+/-)-Methylsuccinic acid dihydroxy-1,4-bis- 9297 (+/-)-Miconazole, nitrate salt (diphenylphosphino)but 9361 (+/-)-Nipecotic acid 6164 (+)-2-Octanol 9618 (+/-)-Phenylpropanolamine .HCl 9110 (+)-6-Methoxy-a-methyl-2- 4923 (+/-)-Sulfinpyrazone naphthaleneacetic acid 10404 (+/-)-Taxifolin 7271 (+)-Amethopterin 4469 (+/-)-Tetrahydropapaveroline .HBr 4368 (+)-Bicuculline 4992 (+/-)-Verapamil, .HCl 7697 (+)-Camphor 9145 (+/-)-a-(Methylaminomethyl)benzyl