Jones Tract Flood Water Quality Investigations Iii
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
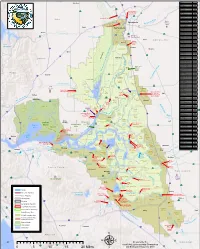
0 5 10 15 20 Miles Μ and Statewide Resources Office
Woodland RD Name RD Number Atlas Tract 2126 5 !"#$ Bacon Island 2028 !"#$80 Bethel Island BIMID Bishop Tract 2042 16 ·|}þ Bixler Tract 2121 Lovdal Boggs Tract 0404 ·|}þ113 District Sacramento River at I Street Bridge Bouldin Island 0756 80 Gaging Station )*+,- Brack Tract 2033 Bradford Island 2059 ·|}þ160 Brannan-Andrus BALMD Lovdal 50 Byron Tract 0800 Sacramento Weir District ¤£ r Cache Haas Area 2098 Y o l o ive Canal Ranch 2086 R Mather Can-Can/Greenhead 2139 Sacramento ican mer Air Force Chadbourne 2034 A Base Coney Island 2117 Port of Dead Horse Island 2111 Sacramento ¤£50 Davis !"#$80 Denverton Slough 2134 West Sacramento Drexler Tract Drexler Dutch Slough 2137 West Egbert Tract 0536 Winters Sacramento Ehrheardt Club 0813 Putah Creek ·|}þ160 ·|}þ16 Empire Tract 2029 ·|}þ84 Fabian Tract 0773 Sacramento Fay Island 2113 ·|}þ128 South Fork Putah Creek Executive Airport Frost Lake 2129 haven s Lake Green d n Glanville 1002 a l r Florin e h Glide District 0765 t S a c r a m e n t o e N Glide EBMUD Grand Island 0003 District Pocket Freeport Grizzly West 2136 Lake Intake Hastings Tract 2060 l Holland Tract 2025 Berryessa e n Holt Station 2116 n Freeport 505 h Honker Bay 2130 %&'( a g strict Elk Grove u Lisbon Di Hotchkiss Tract 0799 h lo S C Jersey Island 0830 Babe l Dixon p s i Kasson District 2085 s h a King Island 2044 S p Libby Mcneil 0369 y r !"#$5 ·|}þ99 B e !"#$80 t Liberty Island 2093 o l a Lisbon District 0307 o Clarksburg Y W l a Little Egbert Tract 2084 S o l a n o n p a r C Little Holland Tract 2120 e in e a e M Little Mandeville -

Transitions for the Delta Economy
Transitions for the Delta Economy January 2012 Josué Medellín-Azuara, Ellen Hanak, Richard Howitt, and Jay Lund with research support from Molly Ferrell, Katherine Kramer, Michelle Lent, Davin Reed, and Elizabeth Stryjewski Supported with funding from the Watershed Sciences Center, University of California, Davis Summary The Sacramento-San Joaquin Delta consists of some 737,000 acres of low-lying lands and channels at the confluence of the Sacramento and San Joaquin Rivers (Figure S1). This region lies at the very heart of California’s water policy debates, transporting vast flows of water from northern and eastern California to farming and population centers in the western and southern parts of the state. This critical water supply system is threatened by the likelihood that a large earthquake or other natural disaster could inflict catastrophic damage on its fragile levees, sending salt water toward the pumps at its southern edge. In another area of concern, water exports are currently under restriction while regulators and the courts seek to improve conditions for imperiled native fish. Leading policy proposals to address these issues include improvements in land and water management to benefit native species, and the development of a “dual conveyance” system for water exports, in which a new seismically resistant canal or tunnel would convey a portion of water supplies under or around the Delta instead of through the Delta’s channels. This focus on the Delta has caused considerable concern within the Delta itself, where residents and local governments have worried that changes in water supply and environmental management could harm the region’s economy and residents. -

Winter Chinook Salmon in the Central Valley of California: Life History and Management
Winter Chinook salmon in the Central Valley of California: Life history and management Wim Kimmerer Randall Brown DRAFT August 2006 Page ABSTRACT Winter Chinook is an endangered run of Chinook salmon (Oncorhynchus tshawytscha) in the Central Valley of California. Despite considerablc efforts to monitor, understand, and manage winter Chinook, there has been relatively little effort at synthesizing the available information specific to this race. In this paper we examine the life history and status of winter Chinook, based on existing information and available data, and examine the influence of various management actions in helping to reverse decades of decline. Winter Chinook migrate upstream in late winter, mostly at age 3, to spawn in the upper Sacramento River in May - June. Embryos develop through summer, which can expose them to high temperatures. After emerging from the spawning gravel in -September, the young fish rear throughout the Sacramento River before leaving the San Francisco Estuary as smolts in January March. Blocked from access to their historical spawning grounds in high elevations of the Sacramento River and tributaries, wintcr Chinook now spawn below Kcswick Dam in cool tail waters of Shasta Dam. Their principal environmental challcnge is temperature: survival of embryos was poor in years when outflow from Shasta was warm or when the fish spawned below Red Bluff Diversion Dam (RBDD), where river temperature is higher than just below Keswick. Installation of a temperature control device on Shasta Dam has reduccd summer temperature in the discharge, and changes in operations of RBDD now allow most winter Chinook access to the upper river for spawning. -

San Joaquin River Riparian Habitat Below Friant Dam: Preservation and Restoration1
SAN JOAQUIN RIVER RIPARIAN HABITAT BELOW FRIANT DAM: PRESERVATION AND RESTORATION1 Donn Furman2 Abstract: Riparian habitat along California's San Joa- quin River in the 25 miles between Friant Darn and Free- Table 1 – Riparian wildlife/vegetation way 99 occurs on approximately 6 percent of its his- corridor toric range. It is threatened directly and indirectly by Corridor Corridor increased urban encroachment such as residential hous- Category Acres Percent ing, certain recreational uses, sand and gravel extraction, Water 1,088 14.0 aquiculture, and road construction. The San Joaquin Trees 588 7.0 River Committee was formed in 1985 to advocate preser- Shrubs 400 5.0 Other riparianl 1,844 23.0 vation and restoration of riparian habitat. The Com- Sensitive Biotic2 101 1.5 mittee works with local school districts to facilitate use Agriculture 148 2.0 of riverbottom riparian forest areas for outdoor envi- Recreation 309 4.0 ronmental education. We recently formed a land trust Sand and gravel 606 7.5 called the San Joaquin River Parkway and Conservation Riparian buffer 2,846 36.0 Trust to preserve land through acquisition in fee and ne- Total 7,900 100.0 gotiation of conservation easements. Opportunities for 1 Land supporting riparian-type vegetation. In increasing riverbottom riparian habitat are presented by most cases this land has been mined for sand and gravel, and is comprised of lands from which sand and gravel have been extracted. gravel ponds. 2 Range of a Threatened or Endangered plant or animal species. Study Area The majority of the undisturbed riparian habitat lies between Friant Dam and Highway 41 beyond the city limits of Fresno. -

Introduction
INTRODUCTION The purpose of this book is twofold: to provide general information for anyone interested in the California islands and to serve as a field guide for visitors to the islands. The book covers both general history and nat- ural history, from the geological origins of the islands through their aboriginal inhabitants and their marine and terrestrial biotas. Detailed coverage of the flora and fauna of one island alone would completely fill a book of this size; hence only the most common, most readily observed, and most interesting species are included. The names used for the plants and animals discussed in this book are the most up-to-date ones available, based on the scientific literature and the most recently published guidebooks. Common names are always subject to local variations, and they change constantly. Where two names are in common use, they are both mentioned the first time the organism is discussed. Ironically, in recent years scientific names have changed more recently than common names, and the reader concerned about a possible discrepancy in nomenclature should consult the scientific literature. If a significant nomenclatural change has escaped our notice, we apologize. For plants, our primary reference has been The Jepson Manual: Higher Plants of California, edited by James C. Hickman, including the latest lists of errata. Variation from the nomenclature in that volume is due to more recent interpretations, as explained in the text. Certain abbreviations used throughout the text may not be immedi- ately familiar to the general reader; they are as follows: sp., species (sin- gular); spp., species (plural); n. -

RD799 Five Year Plan
Reclamation District 799 Five Year Capital Improvement Plan May 2012 Prepared by 2365 Iron Point Road, Suite 300 Folsom, CA 95630 This page intentionally left blank. RD 799 Five Year Plan Contents 1.0 Executive Summary .............................................................................................................. 1 2.0 Brief History of Hotchkiss Tract .......................................................................................... 3 2.1 Location .............................................................................................................................................. 3 2.2 Geomorphic Evolution ........................................................................................................................ 4 2.3 Historical Flood Events ....................................................................................................................... 6 2.3.1 Existing level of protection ........................................................................................................... 6 3.0 Identification of Need for Improvements to Alleviate or Minimize Existing Hazards ........................................................................................................................................ 7 3.1 Local Assets ....................................................................................................................................... 7 3.2 Non-local Assets and Public Benefit .................................................................................................. -

Transitions for the Delta Economy
Transitions for the Delta Economy January 2012 Josué Medellín-Azuara, Ellen Hanak, Richard Howitt, and Jay Lund with research support from Molly Ferrell, Katherine Kramer, Michelle Lent, Davin Reed, and Elizabeth Stryjewski Supported with funding from the Watershed Sciences Center, University of California, Davis Summary The Sacramento-San Joaquin Delta consists of some 737,000 acres of low-lying lands and channels at the confluence of the Sacramento and San Joaquin Rivers (Figure S1). This region lies at the very heart of California’s water policy debates, transporting vast flows of water from northern and eastern California to farming and population centers in the western and southern parts of the state. This critical water supply system is threatened by the likelihood that a large earthquake or other natural disaster could inflict catastrophic damage on its fragile levees, sending salt water toward the pumps at its southern edge. In another area of concern, water exports are currently under restriction while regulators and the courts seek to improve conditions for imperiled native fish. Leading policy proposals to address these issues include improvements in land and water management to benefit native species, and the development of a “dual conveyance” system for water exports, in which a new seismically resistant canal or tunnel would convey a portion of water supplies under or around the Delta instead of through the Delta’s channels. This focus on the Delta has caused considerable concern within the Delta itself, where residents and local governments have worried that changes in water supply and environmental management could harm the region’s economy and residents. -

Comparing Futures for the Sacramento-San Joaquin Delta
comparing futures for the sacramento–san joaquin delta jay lund | ellen hanak | william fleenor william bennett | richard howitt jeffrey mount | peter moyle 2008 Public Policy Institute of California Supported with funding from Stephen D. Bechtel Jr. and the David and Lucile Packard Foundation ISBN: 978-1-58213-130-6 Copyright © 2008 by Public Policy Institute of California All rights reserved San Francisco, CA Short sections of text, not to exceed three paragraphs, may be quoted without written permission provided that full attribution is given to the source and the above copyright notice is included. PPIC does not take or support positions on any ballot measure or on any local, state, or federal legislation, nor does it endorse, support, or oppose any political parties or candidates for public office. Research publications reflect the views of the authors and do not necessarily reflect the views of the staff, officers, or Board of Directors of the Public Policy Institute of California. Summary “Once a landscape has been established, its origins are repressed from memory. It takes on the appearance of an ‘object’ which has been there, outside us, from the start.” Karatani Kojin (1993), Origins of Japanese Literature The Sacramento–San Joaquin Delta is the hub of California’s water supply system and the home of numerous native fish species, five of which already are listed as threatened or endangered. The recent rapid decline of populations of many of these fish species has been followed by court rulings restricting water exports from the Delta, focusing public and political attention on one of California’s most important and iconic water controversies. -

USGS 7.5-Minute Image Map for Clifton Court Forebay, California
O C O A C T S N I O U C U.S. DEPARTMENT OF THE INTERIOR Q CLIFTON COURT FOREBAY QUADRANGLE A A U.S. GEOLOGICAL SURVEY R O CALIFORNIA T J 4 N N 7.5-MINUTE SERIES O Union Island A n█ C 121°37'30" 35' S 32'30" 121°30' 6 000m 6 6 6 6 6 6 6 6 6270000 FEET 37°52'30" 22 E 23 24 25 26 27 28 29 30 37°52'30" C S A O N N T J 6 5 . R . .. O ( . .. 4 ! 3 2 2 . .. 3 .. .. A A C Q U Victoria O I S N Island T C 2140000 A O 4192000mN CAMINO DIABLO C O CAMINO DIABLO FEET 4192 Widdows Island 7 8 10 Eucalyptus 9 10 11 11 Island Old 41 h Riv 91 g Kings er u o l . Island . S ... 4191 . n n .. D a R i l I I a T t T I E . N . O . .. .. .... B . .. .. S CAL PACK RD . .. .. 4190 ... ○ 4190 . ... ... ... .. 14 ... 18 17 Coney Island 16 15 . 15 . .. .. 14 . ... .. .. .. .. .. .. .. .. ... .. .. .. .. .. .. .. ... Clifton Court . .. .. .. .. .. .. .. .. .. .. .. ... .. .. .. .. Forebay . .. .. .. .. .. .. .. ........ ... .. ....... .. .. T1S R4E CLIFTON CT RD 4189 4189 CLIFTON CT . .. .. .... .... Union Island . .... .. .. .. Brushy ... Cr ... .. .. Imagery................................................NAIP, January 2010 Roads..............................................©2006-2010 Tele Atlas Names...............................................................GNIS, 2010 50' 50' Hydrography.................National Hydrography Dataset, 2010 Contours............................National Elevation Dataset, 2010 4188 23 22 HOLEY RD 23 19 20 21 22 23 Byron 41 Byron 88 Airport Airport t 24 c u d e u q A a a i n r o f i l . a . -

Historic, Recent, and Future Subsidence, Sacramento-San Joaquin Delta, California, USA
UC Davis San Francisco Estuary and Watershed Science Title Historic, Recent, and Future Subsidence, Sacramento-San Joaquin Delta, California, USA Permalink https://escholarship.org/uc/item/7xd4x0xw Journal San Francisco Estuary and Watershed Science, 8(2) ISSN 1546-2366 Authors Deverel, Steven J Leighton, David A Publication Date 2010 DOI https://doi.org/10.15447/sfews.2010v8iss2art1 Supplemental Material https://escholarship.org/uc/item/7xd4x0xw#supplemental License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California august 2010 Historic, Recent, and Future Subsidence, Sacramento-San Joaquin Delta, California, USA Steven J. Deverel1 and David A. Leighton Hydrofocus, Inc., 2827 Spafford Street, Davis, CA 95618 AbStRACt will range from a few cm to over 1.3 m (4.3 ft). The largest elevation declines will occur in the central To estimate and understand recent subsidence, we col- Sacramento–San Joaquin Delta. From 2007 to 2050, lected elevation and soils data on Bacon and Sherman the most probable estimated increase in volume below islands in 2006 at locations of previous elevation sea level is 346,956,000 million m3 (281,300 ac-ft). measurements. Measured subsidence rates on Sherman Consequences of this continuing subsidence include Island from 1988 to 2006 averaged 1.23 cm year-1 increased drainage loads of water quality constitu- (0.5 in yr-1) and ranged from 0.7 to 1.7 cm year-1 (0.3 ents of concern, seepage onto islands, and decreased to 0.7 in yr-1). Subsidence rates on Bacon Island from arability. -

9.22 Acre Homesite Off of the San Joaquin River of Sherman Island In
Offering Summary Index • A 9.22 +/- acre homesite off of the San Joaquin River of Sherman Island in Cover .................................................................... 1 Sacramento County, CA • The property presents itself as a great potential for a single-family residence. Offering Summary .......................................2 • Located on the scenic San Joaquin River near Highway 160, North of Antioch. Terms, Rights, & Advisories .................. 3 • Reclamation District No. 341 is in the process of doing levee and road Regional Map .................................................4 enhancements near the property. A letter from the Civil Engineers is on file and available for the Buyers to review at their request. Local Area Map ............................................ 5 Location, Legal Description, & Zoning ........................................................... 6 Property Assesment ................................. 6 Purchase Price & Terms ......................... 6 Soils Map ...........................................................7 Slope Map ........................................................ 8 Assessor Map .................................................9 Photos .............................................................. 10 Page 2 All information contained herein or subsequently provided by Broker has been procured directly from the seller or other third parties and has not been verified by Broker. Though believed to be reliable, it is not guaranteed by seller, broker or their agents. Before entering -

California Regional Water Quality Control Board Central Valley Region Karl E
California Regional Water Quality Control Board Central Valley Region Karl E. Longley, ScD, P.E., Chair Linda S. Adams Arnold 11020 Sun Center Drive #200, Rancho Cordova, California 95670-6114 Secretary for Phone (916) 464-3291 • FAX (916) 464-4645 Schwarzenegger Environmental http://www.waterboards.ca.gov/centralvalley Governor Protection 18 August 2008 See attached distribution list DELTA REGIONAL MONITORING PROGRAM STAKEHOLDER PANEL KICKOFF MEETING This is an invitation to participate as a stakeholder in the development and implementation of a critical and important project, the Delta Regional Monitoring Program (Delta RMP), being developed jointly by the State and Regional Boards’ Bay-Delta Team. The Delta RMP stakeholder panel kickoff meeting is scheduled for 30 September 2008 and we respectfully request your attendance at the meeting. The meeting will consist of two sessions (see attached draft agenda). During the first session, Water Board staff will provide an overview of the impetus for the Delta RMP and initial planning efforts. The purpose of the first session is to gain management-level stakeholder input and, if possible, endorsement of and commitment to the Delta RMP planning effort. We request that you and your designee attend the first session together. The second session will be a working meeting for the designees to discuss the details of how to proceed with the planning process. A brief discussion of the purpose and background of the project is provided below. In December 2007 and January 2008 the State Water Board, Central Valley Regional Water Board, and San Francisco Bay Regional Water Board (collectively Water Boards) adopted a joint resolution (2007-0079, R5-2007-0161, and R2-2008-0009, respectively) committing the Water Boards to take several actions to protect beneficial uses in the San Francisco Bay/Sacramento-San Joaquin Delta Estuary (Bay-Delta).