Pearl Culture
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annual Report 2001-2002, in Which Multiple Activities of Agricultural Research, Education and Extension Are Highlighted
DARE/ICARDARE/ICAR AnnualAnnual ReportReport 2001-20022001-2002 Department of Agricultural Research Indian Council of and Education Agricultural Research Ministry of Agriculture New Delhi Government of India Indian Council of Agricultural Research President Shri Nitish Kumar (Up to 22.7.2001) Minister of Agriculture Shri Ajit Singh (Since 23.7.2001) Minister of Agriculture Vice-President Dr Debendra Pradhan (Up to 1.9.2001) Minister of State (AH&D & DARE) Director-General Dr R S Paroda (Up to 14.8.2001) Secretary Department of Agricultural Research and Education Shri J N L Srivastava (15.8.2001 to 3.10.2001) Secretary, Ministry of Agriculture Dr Panjab Singh (Since 4.10.2001) Secretary Department of Agricultural Research and Education Secretary Smt Shashi Misra (Since 22.2.2001) Additional Secretary Department of Agricultural Research and Education Financial Adviser Shri R S Prasad (Up to 7.6.2001) Joint Secretary and FA Department of Agricultural Research and Education Shri P Sinha (Since 7.6.2001) Additional Secretary and FA Department of Agricultural Research and Education iii OVERVIEW Foreword The National Agricultural Research System (NARS) with the Indian Council of Agricultural Research (ICAR) as an apex body is striving for the holistic development of agriculture at the national level through planning, promoting, conducting and coordinating research, education and extension and training on all aspects of agriculture for ensuring optimal utilization of land, water and plant and animal genetic resources. India has achieved worldwide acclaim in the field of agricultural research, education and extension by achieving more than four-fold increase in foodgrains production besides significant increases in the milk, oilseeds, fruits, vegetables and fish production since independence. -

The Roles of Endolithic Fungi in Bioerosion and Disease in Marine Ecosystems. II. Potential Facultatively Parasitic Anamorphic A
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Dundee Online Publications University of Dundee The roles of endolithic fungi in bioerosion and disease in marine ecosystems. II. Potential facultatively parasitic anamorphic ascomycetes can cause disease in corals and molluscs Gleason, Frank H.; Gadd, Geoffrey M.; Pitt, John I.; Larkum, Anthony W.D. Published in: Mycology DOI: 10.1080/21501203.2017.1371802 Publication date: 2017 Document Version Publisher's PDF, also known as Version of record Link to publication in Discovery Research Portal Citation for published version (APA): Gleason, F. H., Gadd, G. M., Pitt, J. I., & Larkum, A. W. D. (2017). The roles of endolithic fungi in bioerosion and disease in marine ecosystems. II. Potential facultatively parasitic anamorphic ascomycetes can cause disease in corals and molluscs. Mycology, 8(3), 216-227. https://doi.org/10.1080/21501203.2017.1371802 General rights Copyright and moral rights for the publications made accessible in Discovery Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from Discovery Research Portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain. • You may freely distribute the URL identifying the publication in the public portal. Mycology An International Journal on Fungal Biology ISSN: 2150-1203 (Print) 2150-1211 (Online) Journal homepage: http://www.tandfonline.com/loi/tmyc20 The roles of endolithic fungi in bioerosion and disease in marine ecosystems. -

Shelled Molluscs
Encyclopedia of Life Support Systems (EOLSS) Archimer http://www.ifremer.fr/docelec/ ©UNESCO-EOLSS Archive Institutionnelle de l’Ifremer Shelled Molluscs Berthou P.1, Poutiers J.M.2, Goulletquer P.1, Dao J.C.1 1 : Institut Français de Recherche pour l'Exploitation de la Mer, Plouzané, France 2 : Muséum National d’Histoire Naturelle, Paris, France Abstract: Shelled molluscs are comprised of bivalves and gastropods. They are settled mainly on the continental shelf as benthic and sedentary animals due to their heavy protective shell. They can stand a wide range of environmental conditions. They are found in the whole trophic chain and are particle feeders, herbivorous, carnivorous, and predators. Exploited mollusc species are numerous. The main groups of gastropods are the whelks, conchs, abalones, tops, and turbans; and those of bivalve species are oysters, mussels, scallops, and clams. They are mainly used for food, but also for ornamental purposes, in shellcraft industries and jewelery. Consumed species are produced by fisheries and aquaculture, the latter representing 75% of the total 11.4 millions metric tons landed worldwide in 1996. Aquaculture, which mainly concerns bivalves (oysters, scallops, and mussels) relies on the simple techniques of producing juveniles, natural spat collection, and hatchery, and the fact that many species are planktivores. Keywords: bivalves, gastropods, fisheries, aquaculture, biology, fishing gears, management To cite this chapter Berthou P., Poutiers J.M., Goulletquer P., Dao J.C., SHELLED MOLLUSCS, in FISHERIES AND AQUACULTURE, from Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, Eolss Publishers, Oxford ,UK, [http://www.eolss.net] 1 1. -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste -

IJF Front Pages 55-1
EDITORIAL BOARD Dr. S. Ayyappan, Deputy Director General, Dr. Dilip Kumar, Director Indian Council of Agricultural Research, Central Institute of Fisheries Education, Fisheries Division, Krishi Anusandhan Bhavan-II, Jaiprakash Road, Seven Bungalows, Versova, Pusa, New Delhi-110 012. Mumbai-400 061, Maharashtra. Dr. G. Syda Rao Director, Dr. W. S. Lakra, Director Central Marine Fisheries Research Institute, National Bureau of Fish Genetic Resources, P.B. No. 1603, Ernakulam North P.O., Canal Ring Road, Cochin - 682 018, Kerala. P.O. Dilkusha, Lucknow - 226 002, Uttar Pradesh. Dr. K. Devadasan, Director, Central Institute of Fisheries Technology, Dr. A. G. Ponniah, Director Matsyapuri, P.O., Willingdon Island, Cochin - 682 029, Kerala. Central Institute of Brackishwater Aquaculture, 75, Santhome High Road, R.A. Puram, Dr. A. E. Eknath, Director Chennai - 600 028, Tamil Nadu. Central Institute of Freshwater Aquaculture, Dhauli P.O., Kausalyaganga, Dr. K. K. Vass, Director Bhubaneswar - 751 002, Orissa. Central Inland Fisheries Research Institute, Dr. P. C. Mahanta, Director Barrackpore, Kolkata - 743 101, National Research Centre on Cold Water Fisheries, Bhimtal - 263 136, West Bengal. Haldwani, Nainital Dt., Uttar Pradesh. Managing Editor Dr. G. Syda Rao Director, CMFRI, Cochin Editor Dr. Rani Mary George CMFRI, Cochin Sub-Editors Dr. K. S. Sobhana Dr. K. Vinod CMFRI, Cochin CMFRI, Cochin N. Venugopal V. Edwin Joseph CMFRI, Cochin CMFRI, Cochin Publication Committee Dr. N. G. K. Pillai Dr. Rani Mary George Dr. B. Meenakumari CMFRI, Cochin - Member CMFRI, Cochin - Member CIFT, Cochin - Member C. V. Jayakumar CMFRI, Cochin - Member Secretary INDIAN JOURNAL OF FISHERIES Volume 55 : Numbers 1 & 2 January - June 2008 CENTRAL MARINE FISHERIES RESEARCH INSTITUTE (Indian Council of Agricultural Research) COCHIN - 682 018 INDIA Published by The Director, Central Marine Fisheries Research Institute Cochin - 682 018, India On behalf of The Indian Council of Agricultural Research New Delhi - 110 001, India Inland Foreign Annual Subscription Rs. -

Status and Conservation Issues of Window Pane Oyster Placuna Placenta (Linnaeus 1758) in Kakinada Bay, Andhra Pradesh, India
Available online at: www.mbai.org.in doi: 10.6024/jmbai.2015.57.1.1843-15 Status and conservation issues of window pane oyster Placuna placenta (Linnaeus 1758) in Kakinada Bay, Andhra Pradesh, India P. Laxmilatha Central Marine Fisheries Research Institute, P. B. No. 1603, Ernakulum North P.O., Cochin 682 018, Kerala, India. *Correspondence e-mail: [email protected] Received: 27 Apr 2015, Accepted: 27 Jun 2015, Published: 30 Jun 2015 Short Communication Abstract Introduction The Kakinada Bay in Andhra Pradesh, India is a rich ground of the pearl bearing window pane oyster, The windowpane oyster, Placuna placenta, also known as Placuna placenta. The total window pane oyster landing “Kapis”, is a bivalve marine mollusc in the family Placunidae. It during 2011-2012 was 461.3 t; the total effort 13,777 is found in the Gulf of Aden, around India, the Malay Peninsula, man days and mean catch per unit effort (CPUE) 29.7 the southern coasts of China and along the northern coasts of kg. The mean landing was 230.7 t and mean effort Borneo to the Philippines (Yonge, 1977). The major producer was 6,889. The window pane oyster is protected under of “kapis” is Phillipines which exported US $ 36 million worth Schedule IV of the Indian Wildlife Protection Act, of “kapis” products between 1986and 1991 (Gallardo et al., 1972; however, clandestine fishing for live as well as 1995). In India, the window pane oyster is distributed in the fossilized shells of the window pane oyster occurs in Gulf of Kutch (Gujarat) (Hornell, 1909a, b; Moses, 1939, 1947; the Kakinada Bay. -

Proceedings of National Seminar on Biodiversity And
BIODIVERSITY AND CONSERVATION OF COASTAL AND MARINE ECOSYSTEMS OF INDIA (2012) --------------------------------------------------------------------------------------------------------------------------------------------------------- Patrons: 1. Hindi VidyaPracharSamiti, Ghatkopar, Mumbai 2. Bombay Natural History Society (BNHS) 3. Association of Teachers in Biological Sciences (ATBS) 4. International Union for Conservation of Nature and Natural Resources (IUCN) 5. Mangroves for the Future (MFF) Advisory Committee for the Conference 1. Dr. S. M. Karmarkar, President, ATBS and Hon. Dir., C B Patel Research Institute, Mumbai 2. Dr. Sharad Chaphekar, Prof. Emeritus, Univ. of Mumbai 3. Dr. Asad Rehmani, Director, BNHS, Mumbi 4. Dr. A. M. Bhagwat, Director, C B Patel Research Centre, Mumbai 5. Dr. Naresh Chandra, Pro-V. C., University of Mumbai 6. Dr. R. S. Hande. Director, BCUD, University of Mumbai 7. Dr. Madhuri Pejaver, Dean, Faculty of Science, University of Mumbai 8. Dr. Vinay Deshmukh, Sr. Scientist, CMFRI, Mumbai 9. Dr. Vinayak Dalvie, Chairman, BoS in Zoology, University of Mumbai 10. Dr. Sasikumar Menon, Dy. Dir., Therapeutic Drug Monitoring Centre, Mumbai 11. Dr, Sanjay Deshmukh, Head, Dept. of Life Sciences, University of Mumbai 12. Dr. S. T. Ingale, Vice-Principal, R. J. College, Ghatkopar 13. Dr. Rekha Vartak, Head, Biology Cell, HBCSE, Mumbai 14. Dr. S. S. Barve, Head, Dept. of Botany, Vaze College, Mumbai 15. Dr. Satish Bhalerao, Head, Dept. of Botany, Wilson College Organizing Committee 1. Convenor- Dr. Usha Mukundan, Principal, R. J. College 2. Co-convenor- Deepak Apte, Dy. Director, BNHS 3. Organizing Secretary- Dr. Purushottam Kale, Head, Dept. of Zoology, R. J. College 4. Treasurer- Prof. Pravin Nayak 5. Members- Dr. S. T. Ingale Dr. Himanshu Dawda Dr. Mrinalini Date Dr. -
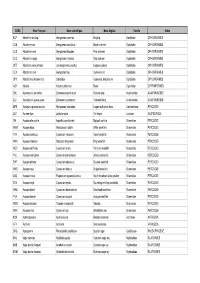
Nom Français
CODE Nom Français Nom scientifique Nom Anglais Famille Ordre KCP Abadèche du Cap Genypterus capensis Kingklip Ophidiidae OPHIDIIFORMES CUB Abadèche noir Genypterus maculatus Black cusk-eel Ophidiidae OPHIDIIFORMES CUS Abadèche rosé Genypterus blacodes Pink cusk-eel Ophidiidae OPHIDIIFORMES CUC Abadèche rouge Genypterus chilensis Red cusk-eel Ophidiidae OPHIDIIFORMES OFZ Abadèche sans jambes Lamprogrammus exutus Legless cuskeel Ophidiidae OPHIDIIFORMES CEX Abadèches nca Genypterus spp Cusk-eels nei Ophidiidae OPHIDIIFORMES OPH Abadèches, brotules nca Ophidiidae Cusk-eels, brotulas nei Ophidiidae OPHIDIIFORMES ALR Ablette Alburnus alburnus Bleak Cyprinidae CYPRINIFORMES ZML Acanthure à pierreries Zebrasoma gemmatum Spotted tang Acanthuridae ACANTHUROIDEI ZLV Acanthure à queue jaune Zebrasoma xanthurum Yellowtail tang Acanthuridae ACANTHUROIDEI MPS Achigan à grande bouche Micropterus salmoides Largemouth black bass Centrarchidae PERCOIDEI LQT Acmée râpe Lottia limatula File limpet Lottiidae GASTROPODA ISA Acoupa aile-courte Isopisthus parvipinnis Bigtooth corvina Sciaenidae PERCOIDEI WEW Acoupa blanc Atractoscion nobilis White weakfish Sciaenidae PERCOIDEI YNV Acoupa cambucu Cynoscion virescens Green weakfish Sciaenidae PERCOIDEI WKK Acoupa chasseur Macrodon ancylodon King weakfish Sciaenidae PERCOIDEI WEP Acoupa du Pérou Cynoscion analis Peruvian weakfish Sciaenidae PERCOIDEI YNJ Acoupa mongolare Cynoscion jamaicensis Jamaica weakfish Sciaenidae PERCOIDEI SWF Acoupa pintade Cynoscion nebulosus Spotted weakfish Sciaenidae PERCOIDEI WKS Acoupa -

Larval Rearing and Spat Production of the Windowpane Shell Placuna Placenta
Research and farming techniques Larval rearing and spat production of the windowpane shell Placuna placenta S. Dharmaraj, K. Shanmugasundaram and C.P. Suja Central Marine Fisheries Research Institute, Tuticorin Research Centre, 115, N.K.Chetty street, Tuticorin-628001 (India), e-mail:[email protected] The windowpane shell, Placuna In Tuticorin Bay, the windowpane and antibiotics were not used during placenta is one among the pearl shell occurs in a 0.46 ha bed and its larval development. We observed the producing bivalves. It was identified as exploitation during the fishery is almost development under an inverted the second-priority mollusc species for total leading to the depletion of stock microscope using 10x10 magnification. research during the Second Conference in the bay. The natural population has After 24 hours straight-hinged larvae on Aquaculture Development in to be augmented in order to meet the were collected in 40µm mesh sieve. The Southeast Asia held in the Philippines needs of mariculture. We therefore larvae were reared in two 75 liter in July 1994 and is the basis for a undertook to develop the technology rectangular fiberglass tanks. Feeding ‘kapis’ (windowpane shell) fishery in for the production of seed of the was initiated at veliger stage on day the Philippines. Windowpane shell species in the hatchery and to sea two. The unicellular microalga provides fishermen an additional ranch them for the replenishment of Isochrysis galbana was given as food income-generating source through the wild stock. The report is the first to be to the larvae once in a day at a sale of its pearls and shells. -

Status of Molluscan Fisheries of India
03 Status of Molluscan Fisheries of India K. Sunil Mohamed Head, Molluscan Fisheries Division Central Marine Fisheries Research Institute Cochin 682018, Kerala. Email: [email protected] Introduction Molluscs are a fascinating and varied group of animals and although their outside features may vary greatly in form and colour, their internal structure are constant. The invertebrate phylum Mollusca with more than 80,000 species is second only to Arthropoda in number of species. In India, the molluscs contribute to important fisheries, providing nutritious food, and are also foreign exchange earners to the country. The shell has many industrial uses and is the object in making eye-catching articles by deft craftsmen. Men, women and children participate in fishing molluscs, which provide employment and income in coastal rural areas. Magnitude of Molluscan Fisheries in India Cephalopods are by far the most important group with an average annual production of about 1,05,000 tonnes (see Fig.1). They are landed as by-catch and as a targeted fishery mostly in mechanized trawlers operating up to 200 m depth, and beyond in some areas. Next in importance are the bivalves and fishing is pursued as a small-scale activity, mostly at subsistence level in various estuaries and inshore seas. The annual average clam production is about 57,000 t, oysters about 18,800 t, and marine mussels about 14,900 t (see Fig.2). There was no fishery for marine pearl oysters since 1962 in the Gulf of Mannar area, which earlier supported major fisheries. Scallops occur in stray numbers and do not form a fishery, while the windowpane oyster was of considerable fishery value till a few years back. -

Shelled Molluscs - Berthou P., Poutiers J.-M., Goulletquer P., Dao J.C
FISHERIES AND AQUACULTURE – Vol. II - Shelled Molluscs - Berthou P., Poutiers J.-M., Goulletquer P., Dao J.C. SHELLED MOLLUSCS Berthou P. Institut Français de Recherche pour l'Exploitation de la Mer, Plouzané, France Poutiers J.-M. Muséum National d’Histoire Naturelle, Paris, France Goulletquer P. Institut Français de Recherche pour l'Exploitation de la Mer, La Tremblade, France Dao J.C. Institut Français de Recherche pour l'Exploitation de la Mer, Plouzané, France Keywords: bivalves, gastropods, fisheries, aquaculture, biology, fishing gears, management Contents 1. Introduction 1.1. Uses of Shellfish: An Overview 1.2. Production 2. Species and Fisheries 2.1. Diversity of Species 2.1.1. Edible Species 2.1.2. Shellfish Species Not Used as Food 2.2. Shelled Molluscs Fisheries 2.2.1. Gastropods 2.2.2. Oysters 2.2.3. Mussels 2.2.4. Scallops 2.2.5. Clams 2.3. Shelled Molluscs Cultivation 2.3.1. Gastropods 2.3.2. Oysters 2.3.3. Mussels 2.3.4. ScallopsUNESCO – EOLSS 2.3.5. Clams 3. Harvesting andSAMPLE Cultivation Techniques CHAPTERS 3.1. Harvesting 3.2. Cultivation techniques 4. Biology 4.1. General Ecology 4.2. Growth 4.3. Reproduction 4.4. Larval Stage in Relation to Dispersal and Stock Abundance 4.5. Migration 5. Stock Assessment and Management Approaches ©Encyclopedia of Life Support Systems (EOLSS) FISHERIES AND AQUACULTURE – Vol. II - Shelled Molluscs - Berthou P., Poutiers J.-M., Goulletquer P., Dao J.C. 5.1. Stock Assessment 5.2. Management Strategies 6. Issues for the Future Bibliography Biographical Sketches Summary Shelled molluscs are comprised of bivalves and gastropods. -

Beyond the Tsunami
Beyond the Tsunami Status of Fisheries in Tamil Nadu, India: A Snapshot of Present and Long-term Trends 2008 A. Murugan and Raveendra Durgekar The Coastal and Marine Programme at ATREE is interdisciplinary in its approach and applies skills in the natural and social sciences to its United Nations Team for Ashoka Trust for Research in Tsunami Recovery Support Ecology and the Environment research and conservation interventions. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the United Nations team for Tsunami Recovery Support (UNTRS), or the United Nations Development Programme (UNDP) concerning the legal status of any country, territory, city or of it authorities or concerning the delimitations of its frontiers or boundaries. Opinion expressed in this publication are those of the authors and do not imply any opinion whatsoever on the part of UNTRS, or UNDP. Copyright © 2008 United Nations India, United Nations Development Programme and Ashoka Trust for Research in Ecology and the Environment Citation Murugan, A. and R. Durgekar. 2008. Beyond the Tsunami: Status of Fisheries in Tamil Nadu, India: A Snapshot of Present and Long-term Trends. UNDP/UNTRS, Chennai and ATREE, Bangalore, India. pp. 75. United Nations team for Tsunami Recovery Support (UNTRS) Apex Towers, 4th floor, 54, 2nd Main Road, R.A. Puram, Chennai-600028, India. Tel:91-44-42303551 www.un.org.in/untrs (valid for the project period only) The United Nations, India 55 Lodi Estate, New Delhi-110003, India. Tel: 91-11-46532262, 46532333 www.un.org.in www.un.org.in/untrs Ashoka Trust for Research in Ecology and the Environment No.