Desert Animal Adaptations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cervid Mixed-Species Table That Was Included in the 2014 Cervid RC
Appendix III. Cervid Mixed Species Attempts (Successful) Species Birds Ungulates Small Mammals Alces alces Trumpeter Swans Moose Axis axis Saurus Crane, Stanley Crane, Turkey, Sandhill Crane Sambar, Nilgai, Mouflon, Indian Rhino, Przewalski Horse, Sable, Gemsbok, Addax, Fallow Deer, Waterbuck, Persian Spotted Deer Goitered Gazelle, Reeves Muntjac, Blackbuck, Whitetailed deer Axis calamianensis Pronghorn, Bighorned Sheep Calamian Deer Axis kuhili Kuhl’s or Bawean Deer Axis porcinus Saurus Crane Sika, Sambar, Pere David's Deer, Wisent, Waterbuffalo, Muntjac Hog Deer Capreolus capreolus Western Roe Deer Cervus albirostris Urial, Markhor, Fallow Deer, MacNeil's Deer, Barbary Deer, Bactrian Wapiti, Wisent, Banteng, Sambar, Pere White-lipped Deer David's Deer, Sika Cervus alfredi Philipine Spotted Deer Cervus duvauceli Saurus Crane Mouflon, Goitered Gazelle, Axis Deer, Indian Rhino, Indian Muntjac, Sika, Nilgai, Sambar Barasingha Cervus elaphus Turkey, Roadrunner Sand Gazelle, Fallow Deer, White-lipped Deer, Axis Deer, Sika, Scimitar-horned Oryx, Addra Gazelle, Ankole, Red Deer or Elk Dromedary Camel, Bison, Pronghorn, Giraffe, Grant's Zebra, Wildebeest, Addax, Blesbok, Bontebok Cervus eldii Urial, Markhor, Sambar, Sika, Wisent, Waterbuffalo Burmese Brow-antlered Deer Cervus nippon Saurus Crane, Pheasant Mouflon, Urial, Markhor, Hog Deer, Sambar, Barasingha, Nilgai, Wisent, Pere David's Deer Sika 52 Cervus unicolor Mouflon, Urial, Markhor, Barasingha, Nilgai, Rusa, Sika, Indian Rhino Sambar Dama dama Rhea Llama, Tapirs European Fallow Deer -

U.S. Fish and Wildlife Service Affirms Protection for Three African Antelope Species Under the ESA
May 31, 2013 Contact: Claire Cassel 703-358-2357 [email protected] U.S. Fish and Wildlife Service Affirms Protection for Three African Antelope Species under the ESA U.S. captive-bred specimens of three endangered African antelope species — the scimitar-horned oryx, dama gazelle and addax – continue to warrant protection under the Endangered Species Act (ESA), the U.S. Fish and Wildlife Service announced today. In findings made in response to two petitions seeking the removal of U.S. captive populations of these endangered species from the Federal List of Endangered and Threatened Wildlife, the Service has determined that providing separate legal status to captive specimens of protected species is not permissible under the ESA. The findings will not impact the current, legal operation of game ranching operations and are consistent with the Service’s longstanding practice for applying the protections of the ESA to captive individuals of species that are threatened or endangered in the wild. The findings were submitted to the Federal Register on May 31. In 2005, the Service added these three antelope species to the Federal List of Endangered and Threatened Wildlife. The species all inhabit the sparse desert regions of northern Africa. Populations for all three species have been greatly reduced as a result of habitat loss, military activity and uncontrolled killing. In the wild, the scimitar-horned oryx may be extirpated, and the other two species are each estimated to number fewer than 500 individuals. Each of the three species occurs in greater numbers in captivity than in the wild, and many of the captive animals are held on private ranches in the United States. -

Addax (Addax Nasomaculatus), Scimitar- Horned Oryx (Oryx Dammah), Arabian Oryx (Oryx Leucoryx), and Sable Antelope (Hippotragus Niger)
Zoo Biology 20:47–54 (2001) Comparisons Among Selected Neonatal Biomedical Parameters of Four Species of Semi-Free Ranging Hippotragini: Addax (Addax nasomaculatus), Scimitar- horned Oryx (Oryx dammah), Arabian Oryx (Oryx leucoryx), and Sable Antelope (Hippotragus niger) Shannon T. Ferrell,1* Robin W. Radcliffe,1 Rodney Marsh,1 Cathy B. Thurman,1 Christine M. Cartwright,1 Thomas W.J. De Maar,2 Evan S Blumer,3 Ed Spevak,4 and Steven A. Osofsky5 1Fossil Rim Wildlife Center, Department of Animal Health Services, Glen Rose, Texas 2Ol Jogi, Ltd., Nanyuki, Kenya 3The Wilds, Cumberland, Ohio 4Wildlife Conservation Society, Bronx, New York 5World Wildlife Fund, Washington, D.C. Basic biomedical data from 164 neonates of four species of the tribe Hippotragini, addax (Addax nasomaculatus), scimitar-horned oryx (Oryx dammah), Arabian oryx (Oryx leucoryx), and sable antelope (Hippotragus niger), were compared at one zoological institution over a 9-year period. Measured biomedical parameters included body weight, temperature, pulse and respiratory rates, packed cell vol- ume (PCV), total plasma protein, glucose, IgG assessment via zinc sulfate tur- bidity, and white blood cell count with differential. All species were maintained in a semi-free ranging setting with the same diet, available shelter, and opportu- nity for social interaction. Based on clinical and field observations, all neonates used in the study were believed to be at least 24 hr old, to have bonded with the dam, and to have no obvious physical abnormalities. Median body weights were similar only in the addax and Arabian oryx with sable antelope having the larg- est median body weight. No significant differences in rectal temperatures or pulse rates were found among species. -

Name Address • Phone Number • Email Address EDUCATION
Name Address • Phone number • Email address EDUCATION_______________________________________________________________________________ • B.S. in Biology with a specialization in Zoology –University (graduated May 2013) • Associate’s of Science – College (graduated May 2010) • Equine Health – College (August 2007 – June 2008) • Culinary Arts Certificate – Career Center (graduated May 2007) • Leadership, Process Improvement, and Team Building Certificate – AAZK (2018) EXPERIENCE_______________________________________________________________________________ Wild Animal Keeper/Temporary Wild Animal Keeper/Replacement Keeper: December 2014 – December 2015; July 2017-December 2017; December 2018-Current Zoo, Place • Responsible for daily husbandry care (diet prep, feeding, cleaning, record-keeping, enrichment, and administering medication) for lemurs, alpaca, capybara, Andean condor, Himalayan tahr, Siberian musk deer, axolotl, cave snakes, seba’s short-tailed bats, straw colored fruit bats, pygmy slow loris, leaf-tailed geckos, spiny stick insects, leafcutter ants, tawny frog mouths, various waterfowl, silver fulu, wood turtle, box turtle, galapagos tortoise, golden lion tamarins, green tree pythons, freshwater stingrays, partula snails, chameleons, naked mole rats, red- eyed tree frogs, mata-mata turtle, and native ohio songbirds. • Operant conditioning for a variety of species • Trained on water quality testing for freshwater and salt water systems Horticulturist/Seasonal Horticulturalist: April 2017-July 2017; December 2017-December 2018 -

Mixed-Species Exhibits with Pigs (Suidae)
Mixed-species exhibits with Pigs (Suidae) Written by KRISZTIÁN SVÁBIK Team Leader, Toni’s Zoo, Rothenburg, Luzern, Switzerland Email: [email protected] 9th May 2021 Cover photo © Krisztián Svábik Mixed-species exhibits with Pigs (Suidae) 1 CONTENTS INTRODUCTION ........................................................................................................... 3 Use of space and enclosure furnishings ................................................................... 3 Feeding ..................................................................................................................... 3 Breeding ................................................................................................................... 4 Choice of species and individuals ............................................................................ 4 List of mixed-species exhibits involving Suids ........................................................ 5 LIST OF SPECIES COMBINATIONS – SUIDAE .......................................................... 6 Sulawesi Babirusa, Babyrousa celebensis ...............................................................7 Common Warthog, Phacochoerus africanus ......................................................... 8 Giant Forest Hog, Hylochoerus meinertzhageni ..................................................10 Bushpig, Potamochoerus larvatus ........................................................................ 11 Red River Hog, Potamochoerus porcus ............................................................... -
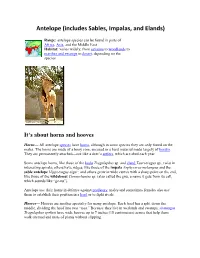
Antelope (Includes Sables, Impalas, and Elands)
Antelope (includes Sables, Impalas, and Elands) Range: antelope species can be found in parts of Africa, Asia, and the Middle East Habitat: varies widely, from savanna to woodlands to marshes and swamps to desert, depending on the species It’s about horns and hooves Horns— All antelope species have horns, although in some species they are only found on the males. The horns are made of a bony core, encased in a hard material made largely of keratin. They are permanently attached—not like a deer’s antlers, which are shed each year. Some antelope horns, like those of the kudu Tragelaphus sp. and eland Taurotragus sp., twist in interesting spirals; others have ridges, like those of the impala Aephyceros melampus and the sable antelope Hippotragus niger; and others grow in wide curves with a sharp point on the end, like those of the wildebeest Connochaetes sp. (also called the gnu, a name it gets from its call, which sounds like “ge-nu”). Antelope use their horns in defense against predators; males and sometimes females also use them to establish their position in a herd or to fight rivals Hooves— Hooves are another specialty for many antelope. Each hoof has a split down the middle, dividing the hoof into two “toes.” Because they live in wetlands and swamps, sitatungas Tragelaphus spekeii have wide hooves up to 7 inches (18 centimeters) across that help them walk on mud and mats of plants without slipping. Nile lechwes Kobus magaceros, which also live in swampy areas, have long, pointed hooves to give them sure footing in the water. -

GNUSLETTER Vol 37#2.Pdf
GNUSLETTER Volume 37 / Number 2 December 2020 ANTELOPE SPECIALIST GROUP IUCN Species Survival Commission Antelope Specialist Group GNUSLETTER is the biannual newsletter of the IUCN Species Survival Commission Antelope Specialist Group (ASG). First published in 1982 by first ASG Chair Richard D. Estes, the intent of GNUSLETTER, then and today, is the dissemination of reports and information regarding antelopes and their conservation. ASG Members are an important network of individuals and experts working across disciplines throughout Africa, Asia and America. Contributions (original articles, field notes, other material relevant to antelope biology, ecology, and conservation) are welcomed and should be sent to the editor. Today GNUSLETTER is published in English in electronic form and distributed widely to members and non-members, and to the IUCN SSC global conservation network. To be added to the distribution list please contact [email protected]. GNUSLETTER Editorial Board - David Mallon, ASG Co-Chair - Philippe Chardonnet, ASG Co-Chair ASG Program Office - Tania Gilbert, Marwell Wildife The Antelope Specialist Group Program Office is hosted and supported by Marwell Wildlife https://www.marwell.org.uk The designation of geographical entities in this report does not imply the expression of any opinion on the part of IUCN, the Species Survival Commission, or the Antelope Specialist Group concerning the legal status of any country, territory or area, or concerning the delimitation of any frontiers or boundaries. Views expressed in GNUSLETTER are those of the individual authors, Cover photo: Young female bushbuck (Tragelaphus scriptus), W National Park and Biosphere Reserve, Niger (© Daniel Cornélis) 2 GNUSLETTER Volume 37 Number 2 December 2020 FROM IUCN AND ASG………………………………………………………. -

Addax Nasomaculatus
Addax nasomaculatus Sand dunes. Great Oriental Erg. Djebil National Park. Tunisia. 2002. © R.C.Beudels, IRScNB Roseline C. Beudels-Jamar, Pierre Devillers, René-Marie Lafontaine and John Newby Institut royal des Sciences naturelles de Belgique 39 ADDAX NASOMACULATUS 1. TAXONOMY AND NOMENCLATURE 1.1. Taxonomy. Addax nasomaculatus belongs to the tribe Hippotragini , sub-family Hippotraginae , family Bovidae , which comprises one extinct species, seven surviving species, and two evolutionary distinct subspecies in genera Oryx, Addax and Hippotragus (Simpson, 1945; Murray, 1984; Corbet et Hill, 1986; Wacher, 1988). All hippotraginids are adapted to the exploitation, generally at low density, of difficult, low-productivity habitats (Kingdon, 1982; Murray, 1984; Wacher, 1988; Beudels, 1993). The genus Addax is comprised of a single species, adapted to the desert. 1.2. Nomenclature. 1.2.1. Scientific name. Female Addax.Termit.1998. Niger. Addax nasomaculatus (De Blainville, 1816). Discribed © Cdt Hama A. Souleymane-DFPP-Niger. as Cerophorus nasomaculata de Blainville, 1816. Bull. Sci. Soc. Philom. Paris, 1816:75. Type locality: None given. Lydekker (1914:148) stated it was “probably Senegambia”, but Grubb (2005) noted that it was more probable that British hunters or collectors obtained Addax from the Tunisian Sahara, to which he restricted the type locality. 1.2.2. Synonyms. Antilope nasomaculatus, Antilope addax, Addax nasomaculatus addax, Antilope naso-maculata, Cerophorus nasomaculata, Antilope suturosa, Antilope mytilopes, Antilope gibbosa, Oryx addax, Oryx naso-maculatus, Addax suturosus, Addax addax 1.2.3. Common names. English : Addax French : Addax, Antilope addax, Antilope de Mendès German: Mendes Antilope Arabic : Begaar el Ouach, Akash, Abu-Akach, Anjidohl, Auel, Bakra el onash, Tamita Tamashek: Amellal Toubou: Turbo 1.2.4. -

The Gazelle in Ancient Egyptian Art Image and Meaning
Uppsala Studies in Egyptology - 6 - Department of Archaeology and Ancient History Uppsala University For my parents Dorrit and Hindrik Åsa Strandberg The Gazelle in Ancient Egyptian Art Image and Meaning Uppsala 2009 Dissertation presented at Uppsala University to be publicly examined in the Auditorium Minus of the Museum Gustavianum, Uppsala, Friday, October 2, 2009 at 09:15 for the degree of Doctor of Philosophy. The examination will be conducted in English. Abstract Strandberg, Åsa. 2009. The Gazelle in Ancient Egyptian Art. Image and Meaning. Uppsala Studies in Egyptology 6. 262 pages, 83 figures. Published by the Department of Archaeology and Ancient History, Uppsala University. xviii +262 pp. ISSN 1650-9838, ISBN 978-91-506-2091-7. This thesis establishes the basic images of the gazelle in ancient Egyptian art and their meaning. A chronological overview of the categories of material featuring gazelle images is presented as a background to an interpretation. An introduction and review of the characteristics of the gazelle in the wild are presented in Chapters 1-2. The images of gazelle in the Predynastic material are reviewed in Chapter 3, identifying the desert hunt as the main setting for gazelle imagery. Chapter 4 reviews the images of the gazelle in the desert hunt scenes from tombs and temples. The majority of the motifs characteristic for the gazelle are found in this context. Chapter 5 gives a typological analysis of the images of the gazelle from offering processions scenes. In this material the image of the nursing gazelle is given particular importance. Similar images are also found on objects, where symbolic connotations can be discerned (Chapter 6). -

The Practical Side of Immunocontraception: Zona Proteins and Wildlife
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Journal of Reproductive Immunology 83 (2009) 151–157 Contents lists available at ScienceDirect Journal of Reproductive Immunology journal homepage: www.elsevier.com/locate/jreprimm The practical side of immunocontraception: zona proteins and wildlife J.F. Kirkpatrick a,∗, A. Rowan b, N. Lamberski c, R. Wallace d, K. Frank a, R. Lyda a a The Science and Conservation Center, ZooMontana, Billings, MT 59106, USA b The Humane Society of the United States, Gaithersburg, MD, USA c San Diego Wild Animal Park, Escondido, CA, USA d Milwaukee County Zoo, Milwaukee, WI, USA article info abstract Article history: With shrinking habitat, the humane control of certain wildlife populations is relevant. The Received 31 December 2008 contraceptive vaccine based on native porcine zona pellucida (PZP) has been applied to Received in revised form 16 June 2009 various wildlife populations for 20 years. Prominent efforts include wild horses, urban Accepted 16 June 2009 deer, zoo animals and African elephants, among others. -

Antelopes, Gazelles, Cattle, Goats, Sheep, and Relatives
© Copyright, Princeton University Press. No part of this book may be distributed, posted, or reproduced in any form by digital or mechanical means without prior written permission of the publisher. INTRODUCTION RECOGNITION The family Bovidae, which includes Antelopes, Cattle, Duikers, Gazelles, Goats, and Sheep, is the largest family within Artiodactyla and the most diverse family of ungulates, with more than 270 recent species. Their common characteristic is their unbranched, non-deciduous horns. Bovids are primarily Old World in their distribution, although a few species are found in North America. The name antelope is often used to describe many members of this family, but it is not a definable, taxonomically based term. Shape, size, and color: Bovids encompass an extremely wide size range, from the minuscule Royal Antelope and the Dik-diks, weighing as little as 2 kg and standing 25 to 35 cm at the shoulder, to the Asian Wild Water Buffalo, which weighs as much as 1,200 kg, and the Gaur, which measures up to 220 cm at the shoulder. Body shape varies from relatively small, slender-limbed, and thin-necked species such as the Gazelles to the massive, stocky wild cattle (fig. 1). The forequarters may be larger than the hind, or the reverse, as in smaller species inhabiting dense tropical forests (e.g., Duikers). There is also a great variety in body coloration, although most species are some shade of brown. It can consist of a solid shade, or a patterned pelage. Antelopes that rely on concealment to avoid predators are cryptically colored. The stripes and blotches seen on the hides of Bushbuck, Bongo, and Kudu also function as camouflage by helping to disrupt the animals’ outline. -

The Future for Wildlife in the Sudan by D
360 The Future for Wildlife in the Sudan By D. C. D. Happold The Government is unaware of the value of wild animals; the people mostly regard them as inferior sorts of cow to be eaten or got rid of; die Game Department struggles to guard them with inadequate staff: what should be done? The author, a zoologist who has spent three years at the University of Khartoum*, surveys the situation, comments on the recommendations in the Fraser Darling Report, and concludes that, if the Sudan is to become one of the principal game countries in Africa, as it should be, some at least of these recommendations must be implemented in the next five to ten years. Sudan is the largest country in Africa, nearly a million square miles. Within its borders are desert, semi-desert, savanna, tropical forest, swamp and mountain. The immense variety of habitat and climate is reflected in the variety of the fauna, and although this has not been studied as extensively as in some other African countries, there are good systematic lists of the mammals24 and birds8, and scattered publications on other groups. The larger fauna, however, is disappearing rapidly, and the last thirty years have seen a big reduc- tion in the numbers and range of many species. It is more urgent now than ever before to conserve these larger animals in national parks and reserves, or by other means. General information about conser- vation and national paries in the Sudan is very limited, especially when compared with East Africa: a recent survey of national parks and reserves in the world by Engelhardt12 gave no details for the Sudan.