Non Steroidal Anti Inflammatory Drugs (NSAIDS)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Horsemen's Information 2016和文TGP 1 薬物修正後 E
[Conditions] 1 Date December 29 (Tue), 2020 2020 Oi Racetrack, Race 10 2 Location TCK, Oi Racetrack 3 Race The 66th Running of Tokyo Daishoten (GI) 4 Eligibility Thoroughbreds, 3 years old & up 5 Full Gate 16 horses 6 Foreign Runners Selected by the selection committee from among the pre-entered horses. 7 Distance 2,000m, 1 1/4 mile (Right-handed, dirt course) 8 Weight 3 years old: 121.5 lbs,4 years old & up: 125.5 lbs, Female: 4.4 lbs less For 3 year-old-horses from the southern hemisphere, reduce 4.4 lbs from the above weight. 9 Purse Unit: 1,000 JPY Prize Purse & Bonus money Running Record 1st place 6th place allowances prize *1 prize *2 1st place 2nd place 3rd place 4th place 5th place or lower Owner 80,000 28,000 16,000 8,000 4,000 300 300 50 1,600 Trainer 880 70 60 50 40 30 30 80 Jockey 120 110 100 80 70 60 30 80 Groom 80 70 60 50 40 30 30 80 Rider 30 30 80 *1 Paid for the runner who broke the previous record and also set the best record during the race. *2 Prize equivalent to the amount listed in the table above is presented. *3 1 USD= 105.88 JPY (As of August,2020) 10 Handling of Late Scratch No allowance is paid in the case of a late scratch (including cancelation of race due to standstill in a starting gate) approved by TCK, Stewards, and Starter. However, if the chairman of the race meeting operation committee deems that the horse is involved in an accident not caused by the horse, the owner is given an running allowance. -

Comparative Study of the Efficacy of Flunixin, Ketoprofen and Phenylbutazone in Delman Horses with Mild Colic
Sys Rev Pharm 2020; 11(5): 464 468 A multifaceted review journal in the field of pharmacy E-ISSN 0976-2779 P-ISSN 0975-8453 Comparative Study of the Efficacy of Flunixin, Ketoprofen and Phenylbutazone in Delman Horses with Mild Colic Agus Purnomo1, Arya Pradana Wicaksono2, Dodit Hendrawan2, Muhammad Thohawi Elziyad Purnama3* 1Department of Veterinary Surgery and Radiology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, DI Yogyakarta, 55281, Indonesia 2Postgraduate Studies, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, 60115, Indonesia 3Department of Veterinary Anatomy, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, 60115, Indonesia *Corresponding author E-mail: [email protected] Article History: Submitted: 26.02.2020 Revised: 16.04.2020 Accepted: 21.05.2020 ABSTRACT This study aimed to evaluate the efficacy of flunixin, ketoprofen and multiple range test. The results showed a significant alleviation in all phenylbutazone on serum biochemistry, plasma catecholamines and observed variables on Day 13, although the use of various NSAIDs serum cortisol in Delman horses with mild colic. During the study showed no significant difference. period, 32 horses were evaluated due to mild colic. Flunixin, Keywords: serum biochemical, catecholamine, cortisol, colic, NSAIDs ketoprofen, and phenylbutazone were administered intravenously at Correspondence: the recommended dose rates of 1.0; 2.2 and 4.4 mg/kg, respectively. Muhammad Thohawi Elziyad Purnama Administration of the NSAIDs commenced on Day 1 and continued Department of Veterinary Anatomy, Faculty of Veterinary Medicine, every 12 h for 12 days. Blood samples collected between days 2, 5, 9 Universitas Airlangga, Surabaya, 60115, Indonesia and 13 to evaluate AST, ALP, GGT, creatinine, urea, epinephrine, E-mail: [email protected] norepinephrine, and cortisol level. -

Non-Steroidal Anti-Inflammatory Drugs (Nsaids)
NON-STEROIDAL ANTI-INFLAMMATORY DRUGS ANALYSIS IN MILK BY QUECHERS AND LC-MS: LOW AND HIGH RESOLUTION DETECTION AND CONFIRMATION APPROACHES A. Rúbies1, L. Guo2, I. Beguiristain1, F. Centrich1, M. Granados2 1. Laboratori Agència de Salut Pública de Barcelona, 2. Departament de Química Analítica - Universitat de Barcelona. * INTRODUCTION NON-STEROIDAL ANTI-INFLAMATORY DRUGS (NSAIDs) Non-steroidal anti-inflammatory drugs (NSAIDs) are used as anti-inflammatory, analgesic and OXICAMS ANTHRANILIC ACID DERIVATIVES ACETIC ACID antipyretic drugs in medicine and veterinary. Their action mechanism is based on the blocking of PROPIONIC ACID DERIVATIVES DERIVATIVES the biosynthesis of prostaglandins. NSAIDs are highly effective and extensively used, but they have some adverse side effects, such as hepatotoxicity, renal disorders or allergic reactions. In the European Union, to assure food safety and protect consumers, maximum residue limits have been established for some authorised NSAIDs in food products. Therefore, high throughput and reliable analytical methodology is required for the effective control of NSAIDs in food from animal Flufenamic acid origin. Liquid chromatography (LC) coupled to mass spectrometry (MS) is currently the technique of choice in confirmatory analysis of NSAIDs residues. We present a new method for the determination of representative NSAIDs in milk based on QuEChERS methodology, LC-MS/MS and UHPLC-HRMS. Meloxicam Ketoprofen Diclofenac EU Maximum Residue Limits (MRLs) Recommended NSAIDs concentrations for NSAIDs in milk. -

Survey of Pain Knowledge and Analgesia in Dogs and Cats by Colombian Veterinarians
veterinary sciences Article Survey of Pain Knowledge and Analgesia in Dogs and Cats by Colombian Veterinarians Carlos Morales-Vallecilla 1, Nicolas Ramírez 1, David Villar 1,*, Maria Camila Díaz 1 , Sandra Bustamante 1 and Duncan Ferguson 2 1 Facultad de Ciencias Agrarias Universidad de Antioquia, Medellín 050010, Colombia; [email protected] (C.M.-V.); [email protected] (N.R.); [email protected] (M.C.D.); [email protected] (S.B.) 2 Department of Comparative Biosciences, College of Veterinary Medicine, University of Illinois at Urbana-Champaign, Urbana, IL 61802, USA; [email protected] * Correspondence: [email protected]; Tel.: +57-3178047381 Received: 6 December 2018; Accepted: 5 January 2019; Published: 10 January 2019 Abstract: A questionnaire study was conducted among 131 veterinarians practicing in the city of Medellin, Colombia, to assess views on pain evaluation and management in dogs and cats. When pain recognition and quantification abilities were used as a perceived competence of proper pain assessment, only 83/131 (63.4%, confidence interval (CI) 0.55–0.72) were deemed to have satisfactory skills, with the rest considered to be deficient. There were 49/131 (37.4) veterinarians who had participated in continuing education programs and were more confident assessing pain, with an odds ratio ( standard error) of 2.84 1.15 (p = 0.01; CI 1.27–6.32). In addition, the odds of using ± ± pain scales was 4.28 2.17 (p < 0.01, CI 1.58–11.55) greater if they had also participated in continuing ± education programs. The term multimodal analgesia was familiar to 77 (58.7%) veterinarians who also claimed to use more than one approach to pain control. -

United States Patent 19 11 Patent Number: 5,366,505 Farber 45 Date of Patent: Nov
O USOO5366505A United States Patent 19 11 Patent Number: 5,366,505 Farber 45 Date of Patent: Nov. 22, 1994 54 METHOD OF REDUCING MEDICAL 4,886,505 12/1989 Haynes et al. ...................... 604/265 DEVICE RELATED INFECTIONS 4,925,668 5/1990 Khan et al. ......................... 424/422 75 Inventor: Bruce Farber, Port Washington, OTHER PUBLICATIONS N.Y. D. G. Maki et al., Clinical Trial of a Novel Antiseptic 73 Assignee: North Shore University Hospital Central Venous Catheter, Abstracts of the 1991 Inter Research Corporation, Manhasset, science Conference on Antimicrobial Agents and Che N.Y. motherapy, p. 176 (1991). C. J. Stephens et al. Randomized Double-Blind Trial 21 Appl. No.: 35,553 Comparing the Risk of Peripheral Vein Thrombophle 22 Filed: Mar. 23, 1993 bitis (T) Between Chlorhexidine (CHA) Coated Cathe ters (C) with Uncoated Control, Abstracts of the 1991 Related U.S. Application Data Interscience Conference on Antimicrobial Agents and Chemotherapy, p. 277 (1991). 63)63 Continuation-in-Tian in-part off Ser. NoNo. 802,891, Dec.ec 6, 1991, M. Tojo et al., Isolation and Characterization of a Cap 5 sular Polysaccharide Adhesin from Staphylococcus epi 51) Int. Cli................................................ A61F 2/02 dermidis, J. Infect. Dis. 157(4): 713-722 (1987). 52 U.S. C. ....................................... 623/11; 604/265 58) Field of Search ........................ 428/413:523/112, Primary Examiner-David Isabella 623/11, 12, 1, 2; 427/2, 604/265 Attorney, Agent, or Firm-Kenyon & Kenyon 56 References Cited 57 ABSTRACT U.S. PATENT DOCUMENTS The growth of microorganisms on catheters and other 4,581,028 4/1986 Fox, Jr. -
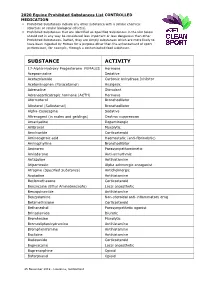
2020 Equine Prohibited Substances List CONTROLLED MEDICATION
2020 Equine Prohibited Substances List CONTROLLED MEDICATION . Prohibited Substances include any other substance with a similar chemical structure or similar biological effect(s). Prohibited Substances that are identified as Specified Substances in the List below should not in any way be considered less important or less dangerous than other Prohibited Substances. Rather, they are simply substances which are more likely to have been ingested by Horses for a purpose other than the enhancement of sport performance, for example, through a contaminated food substance. SUBSTANCE ACTIVITY 17-Alpha-Hydroxy Progesterone FEMALES Hormone Acepromazine Sedative Acetazolamide Carbonic Anhydrase Inhibitor Acetominophen (Paracetamol) Analgesic Adrenaline Stimulant Adrenocorticotropic hormone (ACTH) Hormone Aformoterol Bronchodilator Albuterol (Salbutamol) Bronchodilator Alpha-Casozepine Sedative Altrenogest (in males and geldings) Oestrus suppression Amantadine Dopaminergic Ambroxol Mucolytic Amcinonide Corticosteroid Aminocaproic acid Haemostatic (anti-fibrinolytic) Aminophylline Bronchodilator Aminorex Parasympathomimetic Amiodarone Anti-arrhythmic Antazoline Antihistamine Atipamezole Alpha adrenergic antagonist Atropine (Specified Substance) Anticholinergic Azatadine Antihistamine Beclomethasone Corticosteroid Benzocaine (Ethyl Aminobenzoate) Local anaesthetic Benzquinamide Antihistamine Benzydamine Non-steroidal anti-inflammatory drug Betamethasone Corticosteroid Bethanechol Parasympathetic agonist Brinzolamide Diuretic Bromhexine Mucolytic Bromodiphenhydramine -

An End-To-End Workflow for Quantitative Screening of Multiclass, Multiresidue Veterinary Drugs in Meat Using the Agilent 6470 Triple Quadrupole LC/MS
Application Note Food Testing & Agriculture An End-To-End Workflow for Quantitative Screening of Multiclass, Multiresidue Veterinary Drugs in Meat Using the Agilent 6470 Triple Quadrupole LC/MS Authors Abstract Siji Joseph, Aimei Zou, Chee A comprehensive LC/MS/MS workflow was developed for targeted screening or Sian Gan, Limian Zhao, and quantitation of 210 veterinary drug residues in animal muscle prepared for human Patrick Batoon consumption, with the intention to accelerate and simplify routine laboratory testing. Agilent Technologies, Inc. The workflow ranged from sample preparation through chromatographic separation, MS detection, data processing and analysis, and report generation. The workflow performance was evaluated using three muscle matrices—chicken, pork, and beef— and was assessed on two different Agilent triple quadrupole LC/MS models (an Agilent 6470 and a 6495C triple quadrupole LC/MS). A simple sample preparation protocol using Agilent Captiva EMR—Lipid cartridges provided efficient extraction and matrix cleanup. A single chromatographic method using Agilent InfinityLab Poroshell 120 EC-C18 columns with a 13-minute method delivered acceptable separation and retention time distribution across the elution window for reliable triple quadrupole detection and data analysis. Workflow performance was evaluated based on evaluation of limit of detection (LOD), limit of quantitation (LOQ), calibration curve linearity, accuracy, precision, and recovery, using matrix-matched spike samples for a range from 0.1 to 100 μg/L. Calibration curves were plotted from LOQ to 100 μg/L, where all analytes demonstrated linearity R2 >0.99. Instrument method accuracy values were within 73 to 113%. Target analytes response and retention time %RSD values were ≤19% and ≤0.28% respectively. -

Assessment of the Efficacy of Firocoxib and Robenacoxib
Assessment of the Efficacy of Firocoxib and Robenacoxib in an Induced Synovitis Model of Acute Arthritis in Dogs Christelle Dauteloupa Corinne Pichoub Frederic Beugneta,* aMerial SAS, 2 Av Pasteur, 69007, Lyon, France b Amatsigroup, Site AmatsiAvogadro, Parc de Génibrat, 31470 Fontenilles, France * Corresponding author: E-mail address: [email protected] KEY WORDS: Firocoxib, Robenacoxib, and the increased PVF values compared to Arthritis, Lameness score, Peak Vertical the control group at 3 and 5 hours post- force injection. Firocoxib performed significantly better than robenacoxib at 3, 5, and 10 hours ABSTRACT post-UC injection. In this model, robena- The objective of this study was to compare coxib was not different from control for both the analgesic activity of a single oral dose the VLS and the PVF values. Pre-treatment of two COX-2 selective inhibitors, firo- with firocoxib reduced the induced pain as- coxib (Previcox®, Merial) and robenacoxib sociated with intra-articular administration (Onsior®, Elanco), in an acute pain model of urate crystals. in dogs. Sixteen healthy Beagle dogs were randomly allocated to three groups. Two INTRODUCTION successive experiments were conducted, in Chronic osteoarthritis is common in dogs which eight dogs served as control. Eight and is estimated to affect 20% of dogs dogs received firocoxib or robenacoxib in a over 1 year of age (Johnston, 1997). Since cross-over design at the recommended dos- no drug has been shown to reverse the age 13 hours before intra-articular injection pathological changes of osteoarthritis, the of a urate crystal suspension (UC) for induc- objective of treatment is to reduce pain and tion of synovitis. -

Nonsteroidal Anti-Inflammatory Agents Differ in Their Ability to Suppress
Oncogene (2004) 23, 9247–9258 & 2004 Nature Publishing Group All rights reserved 0950-9232/04 $30.00 www.nature.com/onc Nonsteroidal anti-inflammatory agents differ in their ability to suppress NF-jB activation, inhibition of expression of cyclooxygenase-2 and cyclin D1, and abrogation of tumor cell proliferation Yasunari Takada1, Anjana Bhardwaj1, Pravin Potdar1 and Bharat B Aggarwal*,1 1Cytokine Research Laboratory, Department of Experimental Therapeutics, The University of Texas M.D. Anderson Cancer Center, Box 143, 1515 Holcombe Boulevard, Houston, TX 77030, USA Nonsteroidal anti-inflammatory drugs (NSAIDs) such as Introduction aspirin have been shown to suppress transcription factor NF-jB, which controls the expression of genes such as Owing to its analgesic and anti-inflammatory effects, cyclooxygenase (COX)-2 and cyclin D1, leading to aspirin (acetylsalicylic acid), first synthesized in 1897, inhibition of proliferation of tumor cells. There is no was approved for the treatment of rheumatoid arthritis systematic study as to how these drugs differ in their ability in 1899 (Botting, 1999). Since then several other to suppress NF-jB activation and NF-jB-regulated gene nonsteroidal anti-inflammatory drugs (NSAIDs) have expression or cell proliferation. In the present study, we been synthesized and approved for human use. The anti- investigated the effect of almost a dozen different commonly inflammatory and analgesic effects of NSAIDs have used NSAIDs on tumor necrosis factor (TNF)-induced NF- been shown to be due to their ability to inhibit the jB activation and NF-jB-regulated gene products, and on enzymatic activity of cyclooxygenases (COXs), which cell proliferation. Dexamethasone, an anti-inflammatory convert arachidonic acid to prostaglandins (PGs) (Vane, steroid, was included for comparison with NSAIDs. -

Topical NSAID for Osteoarthritis Safe and Effective (Print)
The Horse: Study: Topical NSAID for Osteoarthritis Safe and Effective (print) Study: Topical NSAID for Osteoarthritis Safe and Effective by: Stacey Oke, DVM, MSc February 26 2009 Article # 13686 Move over, Bute. In a new independent study, researchers at Colorado State University's Gail Holmes Equine Orthopaedic Research Center concluded that diclofenac liposomal cream (1% diclofenac sodium, trade name Surpass) is safer and more effective than phenylbutazone for treating discomfort associated with osteoarthritis in horses. Phenylbutazone, commonly known as "Bute," is a non-steroidal anti-inflammatory (NSAID) drug administered systemically (i.e., intravenously or orally) to help control the pain and inflammation caused by osteoarthritis in horses. "Considering that phenylbutazone and other NSAIDs are known to have important adverse effects in horses when used long-term and that these drugs are not able to alter the course of OA but only help control clinical signs, alternatives are needed," explained researcher David Frisbie, DVM, PhD, Dipl. ACVS. One such alternative is diclofenac liposomal cream--an NSAID that is applied to the skin overlying the affected joint(s) to control pain and inflammation of the tarsal, carpal, metacarpophalangeal, metatarsophalangeal and proximal interphalangeal joints. This product is approved by the Food and Drug Administration and is the first product of its kind manufactured for horses. Results of this study were presented at the 2007 annual American Association of Equine Practitioners' conference and were recently published in the study, "Evaluation of topically administered diclofenac liposomal cream for treatment of horses with experimentally induced osteoarthritis," in the February edition of the American Journal of Veterinary Research. -

Formulations Comprising Nanoparticulate Meloxicam
(19) TZZ¥ZZ¥__T (11) EP 3 090 731 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 09.11.2016 Bulletin 2016/45 A61K 9/14 (2006.01) A61K 31/5415 (2006.01) (21) Application number: 16161176.9 (22) Date of filing: 27.02.2004 (84) Designated Contracting States: • Ryde, Tuula AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Malvern, PA Pennsylvania 19355 (US) HU IE IT LI LU MC NL PT RO SE SI SK TR • Pruitt, John, D. Suwanee, GA Georgia 30024 (US) (30) Priority: 03.03.2003 US 450705 P • Kline, Laura Harleysville, PA Pennsylvania 19438 (US) (62) Document number(s) of the earlier application(s) in accordance with Art. 76 EPC: (74) Representative: Wichmann, Hendrik 08006465.2 / 1 938 803 Wuesthoff & Wuesthoff 04785761.0 / 1 617 816 Patentanwälte PartG mbB Schweigerstraße 2 (71) Applicant: DV Technology LLC 81541 München (DE) Wilmington, Delaware 19801 (US) Remarks: (72) Inventors: This application was filed on 18-03-2016 as a • Cooper, Eugene, R. divisional application to the application mentioned Berwyn, PA Pennsylvania 19312 (US) under INID code 62. (54) FORMULATIONS COMPRISING NANOPARTICULATE MELOXICAM (57) The present invention is directed to composi- than about 2 microns; wherein said effective average par- tions comprising meloxicam. The stable meloxicam com- ticle size is different than the particle size of the nano- positions comprise (a) nanoparticulate particles of mel- particulate particles of meloxicam (a); and (c) at least one oxicam having an effective average particle size of less surface stabilizer. The invention relates also to uses of than about 2000 nm, (b) particles of meloxicam having the composition. -

A. List of Prohibited Substances 1
Reviewed 09.05.17 List over prohibited substances and withdrawal times in Scandinavia, valid from May 20th, 2017 This list has been developed in collaboration with the other Scandinavian countries through NEMAC (Nordic Equine Medication and Anti-doping Committee) The List of prohibited substances and withdrawal times consists of the A-list, listing substances and treatment methods that are absolutely prohibited for horses, and the B-list, listing substances that are prohibited in competition; the withdrawal times for these substances as well as treatment methods with withdrawal times. The list may be reviewed several times per year. This list is valid starting from May 20th, 2017 and is enforced until a new list takes effect. A valid list of withdrawal times can also be found at any time on DNT’s official website, www.travsport.no.ST’s official website, www.travsport.se, and DTC`s official website www.trav.dk Health certificate and keeping of medical records The trainer is responsible for ensuring that any treatment that requires a withdrawal time is listed in the horse’s health certificate. The start and end dates of the treatment, the name of the treatment/medication/active substance, amount given, method of administration, withdrawal time as well as the name of the veterinarian or other person responsible for the treatment must all be listed in the health certificate in accordance with DNT’s Doping Regulations 2017 § 4. Passport and health certificate should be brought with the horse at all times. Omitting, incompletely or improperly listing treatments in the health certificate constitutes a breach of the Doping Regulations.