Peripheral Edema Associated with Calcium Channel Blockers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Interactions Medicamenteuses Index Des Classes Pharmaco
INTERACTIONS MEDICAMENTEUSES INDEX DES CLASSES PHARMACO-THERAPEUTIQUES Mise à jour avril 2006 acides biliaires (acide chenodesoxycholique, acide ursodesoxycholique) acidifiants urinaires adrénaline (voie bucco-dentaire ou sous-cutanée) (adrenaline alcalinisants urinaires (acetazolamide, sodium (bicarbonate de), trometamol) alcaloïdes de l'ergot de seigle dopaminergiques (bromocriptine, cabergoline, lisuride, pergolide) alcaloïdes de l'ergot de seigle vasoconstricteurs (dihydroergotamine, ergotamine, methylergometrine) alginates (acide alginique, sodium et de trolamine (alginate de)) alphabloquants à visée urologique (alfuzosine, doxazosine, prazosine, tamsulosine, terazosine) amidons et gélatines (gelatine, hydroxyethylamidon, polygeline) aminosides (amikacine, dibekacine, gentamicine, isepamicine, kanamycine, netilmicine, streptomycine, tobramycine) amprénavir (et, par extrapolation, fosamprénavir) (amprenavir, fosamprenavir) analgésiques morphiniques agonistes (alfentanil, codeine, dextromoramide, dextropropoxyphene, dihydrocodeine, fentanyl, hydromorphone, morphine, oxycodone, pethidine, phenoperidine, remifentanil, sufentanil, tramadol) analgésiques morphiniques de palier II (codeine, dextropropoxyphene, dihydrocodeine, tramadol) analgésiques morphiniques de palier III (alfentanil, dextromoramide, fentanyl, hydromorphone, morphine, oxycodone, pethidine, phenoperidine, remifentanil, sufentanil) analogues de la somatostatine (lanreotide, octreotide) androgènes (danazol, norethandrolone, testosterone) anesthésiques volatils halogénés -

List of Union Reference Dates A
Active substance name (INN) EU DLP BfArM / BAH DLP yearly PSUR 6-month-PSUR yearly PSUR bis DLP (List of Union PSUR Submission Reference Dates and Frequency (List of Union Frequency of Reference Dates and submission of Periodic Frequency of submission of Safety Update Reports, Periodic Safety Update 30 Nov. 2012) Reports, 30 Nov. -

Health Reports for Mutual Recognition of Medical Prescriptions: State of Play
The information and views set out in this report are those of the author(s) and do not necessarily reflect the official opinion of the European Union. Neither the European Union institutions and bodies nor any person acting on their behalf may be held responsible for the use which may be made of the information contained therein. Executive Agency for Health and Consumers Health Reports for Mutual Recognition of Medical Prescriptions: State of Play 24 January 2012 Final Report Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Acknowledgements Matrix Insight Ltd would like to thank everyone who has contributed to this research. We are especially grateful to the following institutions for their support throughout the study: the Pharmaceutical Group of the European Union (PGEU) including their national member associations in Denmark, France, Germany, Greece, the Netherlands, Poland and the United Kingdom; the European Medical Association (EMANET); the Observatoire Social Européen (OSE); and The Netherlands Institute for Health Service Research (NIVEL). For questions about the report, please contact Dr Gabriele Birnberg ([email protected] ). Matrix Insight | 24 January 2012 2 Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Executive Summary This study has been carried out in the context of Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the application of patients’ rights in cross- border healthcare (CBHC). The CBHC Directive stipulates that the European Commission shall adopt measures to facilitate the recognition of prescriptions issued in another Member State (Article 11). At the time of submission of this report, the European Commission was preparing an impact assessment with regards to these measures, designed to help implement Article 11. -

Ep 0661045 B1
Europäisches Patentamt *EP000661045B1* (19) European Patent Office Office européen des brevets (11) EP 0 661 045 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.7: A61K 9/22, A61K 47/34, of the grant of the patent: A61K 47/36, A61K 9/20 03.07.2002 Bulletin 2002/27 (86) International application number: (21) Application number: 93919648.1 PCT/JP93/01297 (22) Date of filing: 10.09.1993 (87) International publication number: WO 94/06414 (31.03.1994 Gazette 1994/08) (54) SUSTAINED-RELEASE HYDROGEL PREPARATION HYDROGELZUBEREITUNG MIT VERZÖGERTER FREISETZUNG PREPARATION D’HYDROGEL A LIBERATION PROLONGEE (84) Designated Contracting States: • SAWADA, Toyohiro AT BE CH DE DK ES FR GB GR IE IT LI LU NL PT 12-14, Koishigawa-cho 3-chome SE Shizuoka 426 (JP) • OKADA, Akira 12-6, Izumi-cho (30) Priority: 18.09.1992 JP 27497992 Shizuoka 426 (JP) 08.06.1993 JP 16526393 • FUKUI, Muneo 13-14, Minamisurugadai 5-chome Shizuoka 426 (JP) (43) Date of publication of application: 05.07.1995 Bulletin 1995/27 (74) Representative: Geering, Keith Edwin REDDIE & GROSE (73) Proprietor: YAMANOUCHI PHARMACEUTICAL 16 Theobalds Road CO. LTD. London WC1X 8PL (GB) Tokyo 103 (JP) (56) References cited: (72) Inventors: EP-A- 0 067 671 JP-A- 3 002 119 • SAKO, Kazuhiro 7-7, Higashikogawa 4-chome JP-A- 3 034 927 JP-A- 4 217 924 Shizuoka 425 (JP) JP-A- 4 501 411 US-A- 4 968 508 • NAKASHIMA, Hiroshi 28-2, Mise Shizuoka 422 (JP) Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. -

Reversal Effects of Ca2+ Antagonists on Multidrug Resistance Via Down-Regulation of MDR1 Mrna
Kobe J. Med. Sci., Vol. 53, No. 6, pp. 355-363, 2007 Reversal Effects of Ca2+ Antagonists on Multidrug Resistance via Down-regulation of MDR1 mRNA CHIHO KOMOTO1, TSUTOMU NAKAMURA1, 2, MOTOHIRO YAMAMORI1, NOBUKO OHMOTO1, HIRONAO KOBAYASHI3, AKIKO KUWAHARA1, KOHSHI NISHIGUCHI1, KOHJI TAKARA4, YUSUKE TANIGAWARA5, NOBORU OKAMURA2, KATSUHIKO OKUMURA1, 2 and TOSHIYUKI SAKAEDA1, 6 1 Department of Hospital Pharmacy, School of Medicine, Kobe University, Kobe 650-0017, Japan, 2 Department of Clinical Evaluation of Pharmacotherapy, Graduate School of Medicine, Kobe University, Kobe 650-0047, Japan, 3 Shionogi Research Laboratories, Shionogi & Co., Ltd., Osaka 553-0002, Japan, 4 Department of Hospital Pharmacy, Faculty of Pharmaceutical Sciences, Kyoto Pharmaceutical University, Kyoto 607-8414, Japan, 5 Department of Hospital Pharmacy, School of Medicine, Keio University, Tokyo 160-8582, Japan, 6 Center for Integrative Education of Pharmacy Frontier (Frontier Education Center), Graduate School of Pharmaceutical Sciences, Kyoto University, Kyoto 606-8501, Japan Received 21 June 2007 /Accepted 10 September 2007 Key words: MDR1/P-glycoprotein, Ca2+ antagonist, multidrug resistance, down-regulation, mRNA expression In previous reports, the effects of 12 Ca2+ antagonists on a multidrug resistant transporter, P-glycoprotein/MDR1, were evaluated in terms of those on MDR1-mediated transport of [3H]digoxin and the sensitivity of vinblastine sulfate or paclitaxel, and they were able to be classified into 4 subgroups based on their actions, as those with transport inhibition and sensitivity recovery, those with or without transport inhibition but marginal sensitivity recovery, and those without both. In this study, our previous findings were confirmed by the resistance against doxorubicin hydrochloride and daunorubicin hydrochloride, and by the recovery of [3H] vinblastine sulfate accumulation. -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -

Drugs for Primary Prevention of Atherosclerotic Cardiovascular Disease: an Overview of Systematic Reviews
Supplementary Online Content Karmali KN, Lloyd-Jones DM, Berendsen MA, et al. Drugs for primary prevention of atherosclerotic cardiovascular disease: an overview of systematic reviews. JAMA Cardiol. Published online April 27, 2016. doi:10.1001/jamacardio.2016.0218. eAppendix 1. Search Documentation Details eAppendix 2. Background, Methods, and Results of Systematic Review of Combination Drug Therapy to Evaluate for Potential Interaction of Effects eAppendix 3. PRISMA Flow Charts for Each Drug Class and Detailed Systematic Review Characteristics and Summary of Included Systematic Reviews and Meta-analyses eAppendix 4. List of Excluded Studies and Reasons for Exclusion This supplementary material has been provided by the authors to give readers additional information about their work. © 2016 American Medical Association. All rights reserved. 1 Downloaded From: https://jamanetwork.com/ on 09/28/2021 eAppendix 1. Search Documentation Details. Database Organizing body Purpose Pros Cons Cochrane Cochrane Library in Database of all available -Curated by the Cochrane -Content is limited to Database of the United Kingdom systematic reviews and Collaboration reviews completed Systematic (UK) protocols published by by the Cochrane Reviews the Cochrane -Only systematic reviews Collaboration Collaboration and systematic review protocols Database of National Health Collection of structured -Curated by Centre for -Only provides Abstracts of Services (NHS) abstracts and Reviews and Dissemination structured abstracts Reviews of Centre for Reviews bibliographic -

Pharmaceutical Appendix to the Harmonized Tariff Schedule
Harmonized Tariff Schedule of the United States Basic Revision 3 (2021) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States Basic Revision 3 (2021) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

Drug Delivery System for Use in the Treatment Or Diagnosis of Neurological Disorders
(19) TZZ __T (11) EP 2 774 991 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 10.09.2014 Bulletin 2014/37 C12N 15/86 (2006.01) A61K 48/00 (2006.01) (21) Application number: 13001491.3 (22) Date of filing: 22.03.2013 (84) Designated Contracting States: • Manninga, Heiko AL AT BE BG CH CY CZ DE DK EE ES FI FR GB 37073 Göttingen (DE) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO •Götzke,Armin PL PT RO RS SE SI SK SM TR 97070 Würzburg (DE) Designated Extension States: • Glassmann, Alexander BA ME 50999 Köln (DE) (30) Priority: 06.03.2013 PCT/EP2013/000656 (74) Representative: von Renesse, Dorothea et al König-Szynka-Tilmann-von Renesse (71) Applicant: Life Science Inkubator Betriebs GmbH Patentanwälte Partnerschaft mbB & Co. KG Postfach 11 09 46 53175 Bonn (DE) 40509 Düsseldorf (DE) (72) Inventors: • Demina, Victoria 53175 Bonn (DE) (54) Drug delivery system for use in the treatment or diagnosis of neurological disorders (57) The invention relates to VLP derived from poly- ment or diagnosis of a neurological disease, in particular oma virus loaded with a drug (cargo) as a drug delivery multiple sclerosis, Parkinsons’s disease or Alzheimer’s system for transporting said drug into the CNS for treat- disease. EP 2 774 991 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 774 991 A1 Description FIELD OF THE INVENTION 5 [0001] The invention relates to the use of virus like particles (VLP) of the type of human polyoma virus for use as drug delivery system for the treatment or diagnosis of neurological disorders. -
PDF-Document
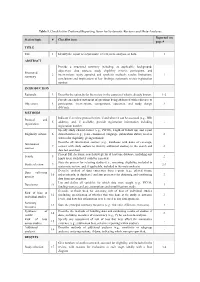
Table 1. Checklist for Preferred Reporting Items for Systematic Reviews and Meta-Analyses. Reported on Section/topic # Checklist item page # TITLE Title 1 Identify the report as a systematic review, meta-analysis, or both. 1 ABSTRACT Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and Structured 2 interventions; study appraisal and synthesis methods; results; limitations; 1 summary conclusions and implications of key findings; systematic review registration number. INTRODUCTION Rationale 3 Describe the rationale for the review in the context of what is already known. 1-2 Provide an explicit statement of questions being addressed with reference to Objectives 4 participants, interventions, comparisons, outcomes, and study design 2 (PICOS). METHODS Indicate if a review protocol exists, if and where it can be accessed (e.g., Web Protocol and 5 address), and, if available, provide registration information including - registration registration number. Specify study characteristics (e.g., PICOS, length of follow-up) and report Eligibility criteria 6 characteristics (e.g., years considered, language, publication status) used as 2 criteria for eligibility, giving rationale. Describe all information sources (e.g., databases with dates of coverage, Information 7 contact with study authors to identify additional studies) in the search and 2 sources date last searched. Present full electronic search strategy for at least one database, including any Search 8 2 limits used, such that it could be repeated. State the process for selecting studies (i.e., screening, eligibility, included in Study selection 9 2-3 systematic review, and, if applicable, included in the meta-analysis). Describe method of data extraction from reports (e.g., piloted forms, Data collection 10 independently, in duplicate) and any processes for obtaining and confirming 3 process data from investigators. -

Wo 2011/060944 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date _ . ... _ 26 May 2011 (26.05.2011) WO 2011/060944 A2 (51) International Patent Classification: (74) Agents: CARVAJAL Y URQUIJO, Isabel et al; A61K 9/16 (2006.01) A61K 9/50 (2006.01) Clarke, Modet & Co., C/Goya 11, 28001 Madrid (ES). A61K 9/48 (2006.01) (81) Designated States (unless otherwise indicated, for every (21) International Application Number: kind of national protection available): AE, AG, AL, AM, PCT/EP20 10/007024 AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (22) International Filing Date: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, 19 November 2010 (19.1 1.2010) HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (25) Filing Language: English KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (26) Publication Langi English NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (30) Priority Data: SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, 200931026 20 November 2009 (20.1 1.2009) ES TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (71) Applicants (for all designated States except US): GP (84) Designated States (unless otherwise indicated, for every PHARM, S.A. [ES/ES]; Poligono Industrial Els Vinyets- kind of regional protection available): ARIPO (BW, GH, Els Fogars, Ctra.