Drinking Water Quality of Rajasthan Districts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
BO GRO LIST UPDATED 20-21-Without Email Id.Xlsx
BRANCH GRIEVANCE REDRESSAL OFFICERS : 2020-21 SL NO Zone Division Branch Name Branch Name of Branch Incharge & Designation Tel No. with STD Code code 1 NORTHERN ZONE AJMER AJMER-2 106 Shri Rajeev Sharma 0145-2662465 2 NORTHERN ZONE AJMER KOTA-2 109 Shri Prameet billiyan 0744-2473051 3 NORTHERN ZONE AJMER AJMER-1 181 Sh. Manoj Kumar 0145-2429756 4 NORTHERN ZONE AJMER KOTA-1 182 Shri Ajay Goyal 0744-2390457 5 NORTHERN ZONE AJMER BHILWARA-1 198 Shri khem Raj Meena 01482-239838 6 NORTHERN ZONE AJMER KOTA-CAB 313 Shri K C Mahavar 0744-2390797 7 NORTHERN ZONE AJMER BEAWAR 321 Shri Kailash Chand 01462-225039 8 NORTHERN ZONE AJMER JHALAWAR 322 Shri B R Meena 07432-232433 9 NORTHERN ZONE AJMER LAKHAERI 1201 Shri Manish Kapoor 07438-261681 10 NORTHERN ZONE AJMER KEKRI 1202 SH. Suresh Chandra Meena 01467-222475 11 NORTHERN ZONE AJMER BUNDI 18A Shri V D Singh 0747-2456620 12 NORTHERN ZONE AJMER KISHANGARH 18F Shri Kailash Kumar Meena 01463-246775 13 NORTHERN ZONE AJMER SHAHAPURA 18J Shri S K Srivastava 01482-223564 14 NORTHERN ZONE AJMER BARAN 18M Shri P L Meena 07453-230165 15 NORTHERN ZONE AJMER NASIRABAD 18N Shri AD Tiwari 01491-220273 16 NORTHERN ZONE AJMER BHILWARA-2 18U Shri R D Dad 01482-243199 17 NORTHERN ZONE AJMER KOTA-3 18X Shri Pawan Kumar 0744-2472423 18 NORTHERN ZONE AJMER AJMER CAB 29N Shri J N Bairwa / SBM 0145-2664665 19 NORTHERN ZONE AJMER BHAWANI MANDI 29P shri Rajesh Kumar Rathi 07433-222725 20 NORTHERN ZONE AJMER BIJAYNAGAR 29R Shri K.S.Meena 01462-230149 21 NORTHERN ZONE AMRITSAR Pathankot-I 135 Sh. -

Brief Industrial Profile of Karauli District
lR;eso t;rs Government of India Ministry of MSME Brief Industrial Profile of Karauli District Carried out by MSME-Development Institute (Ministry of MSME, Govt. of India,) 22 Godam, Industrial Estate, Jaipur-302006 Phone: 0141-2212098, 2213099 Fax: 0141-2210553 E-mail: [email protected] Web- www.msmedijaipur.gov.in 1 2 Contents S. No. Topic Page No. 1. General Characteristics of the District 4 1.1 Location & Geographical Area 4 1.2 Topography 4 1.3 Availability of Minerals. 4 1.4 Forest 5 1.5 Administrative set up 5 2. District at a glance 5-7 2.1 Existing Status of Industrial Area 7 3. Industrial Scenario 3.1 Industry at a Glance 8 3.2 Year Wise Trend Of Units Registered 8 3.3 Details Of Existing Micro & Small Enterprises & Artisan Units In The 9 District 3.4 Large Scale Industries / Public Sector undertakings 9 3.5 Major Exportable Item 9 3.6 Growth Trend 9 3.7 Vendorisation / Ancillarisation of the Industry 9 3.8 Medium Scale Enterprises 10 3.8.1 List of the units 10 3.8.2 Major Exportable Item 10 3.9 Service Enterprises 10 3.9.2 Potentials areas for service industry 10 3.10 Potential for new MSMEs 10 4. Existing Clusters of Micro & Small Enterprise 10 4.1 Detail Of Major Clusters 10 4.1.1 Manufacturing Sector 10 4.1.2 Service Sector 11 4.2 Details of Identified cluster 11 4.2.2 Stone cluster 11 5. General issues raised by industry association during the course of 11 meeting 6 Steps to set up MSMEs 12 7. -
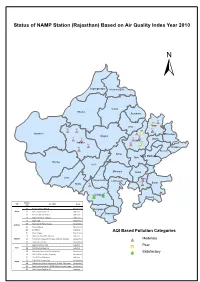
Rajasthan NAMP ARCGIS
Status of NAMP Station (Rajasthan) Based on Air Quality Index Year 2010 ± Sriganganager Hanumangarh Churu Bikaner Jhunjhunu 219 373 *# Alwar(! Sikar 274 273 372 297 *# *# 409 *# Jaisalmer *# (! Bharatpur Nagaur 408 376 410 411 *# Dausa *# *# *#Jaipur 296 Jodhpur 298 412 *# (! 413 *# Dholpur *# Karauli Ajmer Sawai Madhopur Tonk Barmer Pali Bhilwara Bundi *#326 Jalor Kota# Rajsamand Chittorgarh * 325 17 Baran Sirohi *#321 *# 294 320Udaipurjk jk Jhalawar Station City Location code Area 372 Regional Office,RSPCB Residential Dungarpur Alwar 373 M/s Gourav Solvex Ltd Industrial Banswara 219 RIICO Pump House MIA Industrial 274 Regional Office, Jodhpur Industrial 273 Sojati Gate Residential 376 Mahamandir Police Thana Residential Jodhpur 411 Housing Board Residential 413 DIC Office Industrial AQI Based Pollution Categories 412 Shastri Nagar Residential 321 Regional Office MIA, Udaipur Industrial Udaipur 320 Ambamata, Udaipur (Chandpur Sattllite Hospital) Residential *# Moderate 294 Town Hall, Udaipur Residential 17 Regional Office, Kota Industrial Poor Kota 325 M/s Samcore Glass Ltd Industrial (! 326 Municipal Corporation Building, Kota Residential Satisfactory 298 RSPCB Office, Jhalana Doongari Residential jk 410 RIICO Office MIA, Jaipur Industrial 296 PHD Office, Ajmeri Gate Residential Jaipur 408 Office of the District Educational Officer, Chandpole Residential 409 Regional Office North, RSPCB,6/244 Vidyadhar Nagar Residential 297 VKIA, Jaipur (Road no.-6) Industrial Status of NAMP Station (Rajasthan) Based on Air Quality Index Year 2011 ± -

District Profile Pali, Rajasthan
District Profile Pali, Rajasthan Pali District has an area of 12,387 km². The district lies between 24° 45' and 26° 29' north latitudes and 72°47' and 74°18' east longitudes. The Great Aravali hills link Pali district with Ajmer, Rajsamand, Udaipur and Sirohi Districts. The district has 10 blocks, as recorded in 2014—Jaitaran, Raipur, Sojat, Rohat, Pali, Marwar Junction, Desuri, Sumerpur and Bali. DEMOGRAPHY As per Census 2011, the total population of Pali is 2037573. The percentage of urban population in Pali is 22.6 percent. Out of the total population there are 1025422 males and 1012151 females in the district. This gives a sex ratio of 987 females per 1000 males. The decadal growth rate of population in Rajasthan is 21.31 percent, while Pali reports a 11.94 percent of decadal increase in the population. The district population density is 164 in 2011. The Scheduled Caste popula- tion in the district is 19.53 percent while Scheduled Tribe comprises 7.09 percent of the population. LITERACY The overall literacy rate of district is 62.39 percent while the male & female literacy rate is 76.81 and 48.01 percent respectively. At the block level, a con- siderable disparity is noticeable in the male-female literacy rate. Pali block has the highest male literacy rate of 82.56 percent and female literacy rate of 57.09 percent. Similarly, the lowest male and female literacy rate is found in Bali (71.58 percent) and Jaitaran (41.62 percent) blocks respectively. Source: Census 2011 A significant difference is notable in the literacy rate of rural and urban Pali. -

Census Report of Karauli State, Rajasthan
CENSUS REPORT OF KARAULI STATE 1931 BY Babu Kiatoor Chand dain, B. A., Census Superintendent, KARAULISTATE, RAJPUTANA. LUCKNOW: PRINTED BY K. D. SETH AT THE NEWVL KISHORE PRESS, 1.933. TABLE OF CONTENTS. PAca:. Ihtroducti{)n '0. I-IV Chapter J. Distribution and movement o~ the population. SUbject-ma.tter ... \-1\ Subsidiary tables ... 7-11 Statement of rainfall. cultivated area 1~-13 Chapter II. Population o~ cities, to",,""a and villages. Subject-ma.tter ... H,-Hi SubAidiary table!' 1!J-':U Chapter III. Birth-place and migration. Subject-matter ... Subsidiary tables Chapter IV. Age. Subject-IDS.tter 27-31 Subsidiary tables 32-4-2 Chapter v. Sex. Subject-matter Subsidiary tables Chapter VI. Civil Condition. Subject-matte!' Subsidiary tables Chapter VII. Infirmities. Subject-matter 64-67 Subsidiary tables 68-71 Chapter VIII. Occupation. Subject-matter 72-83 Subsidiary ta.bles 84-104 Chapter IX. Literacy. Subject·matter 105-109 Snbaidia.-ry tableR }, \ 'C<-l.l'3 Chapter x. Language. Subject-matter 114-115 Subsidiary tables 116-11'1 Chapter XI. Religion. S11bject-mattel' US-UJO Subsidiary tables 1!U-l!i!8 Chapter XII. Ra.ce. Tribe and Caste. Subjectrmattel' 1~<&-li6 Subsidiary tablos ... ... UI7-18!1 PROVINCIAL TABLES. PAGE- Tabl. I. Area and Population 138 Table II_ Population of districts by religion and literacy 1340 Table III. Caste 135 Table I V. Language 186 Il\IIPERIAL TABLES. Table I. Area-HouBes and popUlation Karauli State, 1931 137 Table II. Variation in population during the last 50 years 138 Table JII. ToWDS and villages classified by population 139 Table IV. -

A CASE STUDY of SIKAR, RAJASTHAN Manisha
Journal of Global Resources Volume 4 (01) January 2018 Page 133-137 ISSN: 2395-3160 (Print), 2455-2445 (Online) 20 STRATEGIC PLANS FOR INDUSTRIAL DEVELOPMENT AND ITS POTENTIAL: A CASE STUDY OF SIKAR, RAJASTHAN Manisha Sharma 1and M. A. Khan 2 1 Head, Dept. of Geography. B.D. Todi College, Lachhmangarh (Sikar), India 2Deputy Director, Department of Minority Affairs, GoR, Jaipur, Rajasthan, India Abstract: Industrial Potential means anything that may be a possibility; potentially and existing in possibility, not in actuality and being potent. Industrial or economic potential is determined by the quantity of labor resources and the quality of their vocational training by the volume of production capacities of Industrial and construction organizations, by the production capacities of agriculture, by the extent of transportation arteries, by the development of sectors in the non-production sphere by advances in science and technology and by the resources of explored mineral deposits. Resources have the most vital role in the development of a region. The main resources of study area which have great significance for industrial development are minerals, agriculture and livestock. Water and electricity provides basis for Industrial development. However, the area has good potential for agro based, mineral based and livestock based industries. As Sikar enjoys the benefits of surplus agricultural production and large number of livestock, it would be worthwhile to propose few agro based and livestock industrial units. Limestone is also being exploited so, there is scope of cement and others industries. This paper describes a strategic plan for development of Industries and its potential in Sikar district. -

Government of India Ministry of Human Resource Development Department of School Education and Literacy ***** Minutes of the Meet
Government of India Ministry of Human Resource Development Department of School Education and Literacy ***** Minutes of the meeting of the Project Approval Board held on 14th June, 2018 to consider the Annual Work Plan & Budget (AWP&B) 2018-19 of Samagra Shiksha for the State of Rajasthan. 1. INTRODUCTION The meeting of the Project Approval Board (PAB) for considering the Annual Work Plan and Budget (AWP&B) 2018-19 under Samagra Shiksha for the State of Rajasthan was held on 14-06-2018. The list of participants who attended the meeting is attached at Annexure-I. Sh Maneesh Garg, Joint Secretary (SE&L) welcomed the participants and the State representatives led by Shri Naresh Pal Gangwar, Secretary (Education), Government of Rajasthan and invited them to share some of the initiatives undertaken by the State. 2. INITIATIVES OF THE STATE Adarsh and Utkrisht Vidyalaya Yojana: An Adarsh Vidyalaya (KG/Anganwadi-XII) has been developed in each Gram Panchayat as center of excellence. An Utkrisht Vidyalaya (KG/Anganwadi-VIII) has also been developed in each Gram Panchayat under the mentorship of Adarsh school to ensure quality school coverage for other villages in the Gram Panchayat. Panchayat Elementary Education Officer- Principals of Adarsh school have been designated as ex-officio Panchayat Elementary Education Officer (PEEO) to provide leadership and mentorship to all other government elementary schools in the Gram Panchayat. These PEEOs have been designated as Cluster Resource Centre Facilitator (CRCF) for effective monitoring. Integration of Anganwadi centers with schools- Around 38000 Anganwadi centers have been integrated with schools having primary sections for improving pre-primary education under ECCE program of ICDS. -

State: RAJASTHAN Agriculture Contingency Plan for District : SIKAR
State: RAJASTHAN Agriculture Contingency Plan for District : SIKAR 1.0 District Agriculture Profile 1.1 Agro-Climatic/Ecological Zone Agro Ecological Sub Region (ICAR) Western Plain, Kachchh And Part Of Kathiawar Peninsula, Hot Arid Eco-Region (2.3) Agro-Climatic Zone (Planning Commission) Western Dry Region (XIV) Agro climatic zone (NARP)* Transitional Plain Of Inland Drainage Zone (RJ-3) List all the districts falling under the NARP zone Sikar, Jhunjhunu, Nagaur and parts of Churu. Geographic coordinates of district Latitude Longitude Altitude 0 0 0 o 27 21’ N to 28 12’ N 74 44 ‘ E to 75 25’E 341.0 (m) Name and address of the concerned Agriculture Research Station ZRS/ZARS/RARS/RRS/RRTTS Fatehpur-shekhawati, Distt.:Sikar (Raj.) 332301 Mention the KVK located in the district One (Fatehpur-Shekhawati, Sikar) 1.2 Rainfall Average(mm) Normal onset Normal cessation (specify week and month) (specify week and month) SW monsoon (June-sep.) 364.0 Last week of June Last week of September NE monsoon (oct.-dec.) - - - Winter (Jan-March) - 1 Summer (Apr-may) - Annual 364.0 1.3 Land Geographic Cutivable Forest Land under Parmanent Cultivable Land under Barren&un Current usePattern of al area area non wasteland misc.tree cultivable the distt. area agriculture Past. crops fallows &groves land use Area(000” ha) 774 531.3 61.08 33.93 40.640 38.14 .06 18.24 9.21 1.4 Major soils Area(000 ha) Percent(%) of total Sandy soils 379.7 49.0 Fertile soils (Sandy loam) 394.4 50.9 1.5 Agriculture land use Area(000 ha) Cropping intensity % Net sown area 522.3 140.6 Area sown more than once 212.4 Gross cropped area 734.2 1.6 Irrigation Area(000 ha) Net cultivated area 610.7 2 Net irrigated area 262.6 Gross cultivated area 734.7 Gross irrigated area 266.1 Rainfed area 622.3 Sources of irrigation Number Area(000 ha) % area Canals - - Tanks - - - Open wells & Bore well ( No.) 45475 262.6 100 Lift irrigation - - - Other sources - - - Total - 262.6 100 Pump sets - - - Micro irrigation - - - Groundwater availability and use No. -

THEIR OWN COUNTRY :A Profile of Labour Migration from Rajasthan
THEIR OWN COUNTRY A PROFILE OF LABOUR MIGRATION FROM RAJASTHAN This report is a collaborative effort of 10 civil society organisations of Rajasthan who are committed to solving the challenges facing the state's seasonal migrant workers through providing them services and advocating for their rights. This work is financially supported by the Tata Trust migratnt support programme of the Sir Dorabji Tata Trust and Allied Trusts. Review and comments Photography Jyoti Patil Design and Graphics Mihika Mirchandani All communication concerning this publication may be addressed to Amrita Sharma Program Coordinator Centre for Migration and Labour Solutions, Aajeevika Bureau 2, Paneri Upvan, Street no. 3, Bedla road Udaipur 313004, Ph no. 0294 2454092 [email protected], [email protected] Website: www.aajeevika.org This document has been prepared with a generous financial support from Sir Dorabji Tata Trust and Allied Trusts In Appreciation and Hope It is with pride and pleasure that I dedicate this report to the immensely important, yet un-served, task of providing fair treatment, protection and opportunity to migrant workers from the state of Rajasthan. The entrepreneurial might of Rajasthani origin is celebrated everywhere. However, much less thought and attention is given to the state's largest current day “export” - its vast human capital that makes the economy move in India's urban, industrial and agrarian spaces. The purpose of this report is to bring back into focus the need to value this human capital through services, policies and regulation rather than leaving its drift to the imperfect devices of market forces. Policies for labour welfare in Rajasthan and indeed everywhere else in our country are wedged delicately between equity obligations and the imperatives of a globalised market place. -

Sharma, V. & Sankhala, K. 1984. Vanishing Cats of Rajasthan. J in Jackson, P
Sharma, V. & Sankhala, K. 1984. Vanishing Cats of Rajasthan. J In Jackson, P. (Ed). Proceedings from the Cat Specialist Group meeting in Kanha National Park. p. 116-135. Keywords: 4Asia/4IN/Acinonyx jubatus/caracal/Caracal caracal/cats/cheetah/desert cat/ distribution/felidae/felids/Felis chaus/Felis silvestris ornata/fishing cat/habitat/jungle cat/ lesser cats/observation/Prionailurus viverrinus/Rajasthan/reintroduction/status 22 117 VANISHING CATS OF RAJASTHAN Vishnu Sharma Conservator of Forests Wildlife, Rajasthan Kailash Sankhala Ex-Chief Wildlife Warden, Rajasthan Summary The present study of the ecological status of the lesser cats of Rajasthan is a rapid survey. It gives broad indications of the position of fishing cats, caracals, desert cats and jungle cats. Less than ten fishing cats have been reported from Bharatpur. This is the only locality where fishing cats have been seen. Caracals are known to occur locally in Sariska in Alwar, Ranthambore in Sawaimadhopur, Pali and Doongargarh in Bikaner district. Their number is estimated to be less than fifty. Desert cats are thinly distributed over entire desert range receiving less than 60 cm rainfall. Their number may not be more than 500. Jungle cats are still found all over the State except in extremely arid zone receiving less than 20 cms of rainfall. An intelligent estimate places their population around 2000. The study reveals that the Indian hunting cheetah did not exist in Rajasthan even during the last century when ecological conditions were more favourable than they are even today in Africa. The cats are important in the ecological chain specially in controlling the population of rodent pests. -

Project Technical Report
Project Technical Report Motivation of communities for wildlife conservation (Blackbuck) in and outside the protected areas in Western Rajasthan Project Investigator Dr. HEMSINGH GEHLOT Sponsored by: Year -2010 SAVE THE BLACKBUCK Copyright © Hemsingh Gehlot This report may be quoted freely but the source must be acknowledged and to be cited as: Gehlot, H.S. (2010) Motivation of communities for wildlife conservation (Blackbuck) in and outside the protected areas in Western Rajasthan Report copy can be obtained from: The Rufford Maurice Laing Foundation Dr. HEMSINGH GEHLOT “ Sankalp” 5th Floor Babmaes House, 80, Chaturawata, Chainpura 2 Babmaes Street, Mandore, Jodhpur - 342304 Landon Rajasthan (INDIA) SW1Y 6RD Email: [email protected] Email: [email protected] Web: www.rufford.org/rsg Photo credits: Hemsingh Gehlot 2 Contents Page No. Acknowledgements 4 Introduction 5 Project Objectives and Study area 3 Methodology and Field Survey 4 Major threats for Blackbuck and its habitat 9 Motivation of communities for wildlife conservation through awareness 11 Recommendations and Future plan 13 References 14 Project team 16 Annexure I Distribution of Blackbuck at Taluka level in western Rajasthan Annexure II Project news in local media Annexure III Media clip showing the status of Blackbuck mortality in Rajasthan Annexure IV Inauguration of awareness material Annexure V Campaign Brochure and pamphlet Annexure VI Photo Documentation 3 Acknowledgements It is a pleasure for me to acknowledge the help, which I received during this fieldwork and thereafter in preparing technical report. Execution of this project was made possible due to the financial support by ‘Rufford Small Grant Program, UK’. I therefore express sincere gratitude on the behalf of my whole team to RSG especially to Mr. -

List of Private Empaneled Hospitals S.N
List of Private Empaneled Hospitals S.N. District Hosp_Code Hospital Name Address H_INCHARGE Name MOBILE NO. Email _Id 1 AJMER Ajme386 ANAND MULTISPECIALITY & RESEARCH GOVINDPURA, JALIYA ROAD, JODHPUR DR. NARENDRA 9269625000 [email protected], CENTER BYE PASS, BEAWAR ANANDANI 2 AJMER Ajme1226 DEEPMALA PAGARANI HOSPITAL & 76-A, INSIDE SWAMI MADHAV DWAR, DR. JAWAHAR LAL 0145-2445445, [email protected], RESEARCH CENTRE ADARSH NAGAR GARGIYA 9414444491, 9414148424 3 AJMER Ajme1650 DR KHUNGER EYE CARE HOSPITAL OPP PNB BANK DR. NEERAJ KHUNGER 0145-2442000, [email protected] 9982537977, 9829070265 4 AJMER Ajme1882 DR VIJAY ENT HOSPITAL 58 59 MAKARWALI ROAD ST STEPHEN DR.VIJAY GAKHAR 9982147067 [email protected] CIRCLE VAISHALI NAGAR AJMER 5 AJMER Ajme168 JMD HOSPITAL & RESEARCH CENTRE HIGHWAY COLONY, BEAWAR DR. ASHA KHANNA 01462-252290, [email protected], 9829071475 6 AJMER Ajme821 KHETRAPAL HOSPITAL SECTOR C, PANCHSHEEL NAGAR ALOK SHARMA 0145-2970501, [email protected], MULTISPECIALITY & RESEARCHJ CENTRE 9116010913 7 AJMER Ajme1799 MARBLE CITY HOSPITAL KISHANGARH ANKIT SHARMA 9694090058 [email protected] 8 AJMER Ajme1144 MEWAR HOSPITAL PVT LTD A-175, HARI BHAU UPADHYAY NAGAR SAIF MOIN 0145-2970188, [email protected], MAIN, NEAR GLITZ CINEMA 7727009307 9 AJMER Ajme1316 MITTAL HOSPITAL & RESEARCH CENTER PUSHKAR ROAD YUVRAJ PARASHAR 9351415247 [email protected], 10 AJMER Ajme1653 PUSHPA CHANDAK MEMORIAL BANGALI GALI, KUTCHERY ROAD DR. LAXMI NIWAS 0145-2626208, [email protected], MULTISPECIALITY HOSPITAL CHANDAK 9829087032 11 AJMER Ajme822 RATHI HOSPITAL AJMER ROAD, KISHANGARH DR. SANJAY RATHI 01463-247050, [email protected], 9166915452 12 AJMER Ajme1694 SHREE PARSHVNATH JAIN HOSPITAL UDAIPUR ROAD,BEAWAR DR NM SINGHVI 01462-223351, [email protected] AND RESEARCH CENTER 9414277764 13 AJMER Ajme1401 SHREE PKV (PRAGYA KUNDAN BEAWAR ROAD, BIJAINAGAR DR.