EMERGING THREAT REPORT Third Quarter 2020 Special Testing and Research Laboratory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Report of the International Narcotics Control Board for 2020 (E/INCB/2020/1) Is Supplemented by the Following Reports
INTERNATIONAL NARCOTICS CONTROL BOARD Report 2020 EMBARGO Observe release date: Not to be published or broadcast before Thursday 25 March 2021, at 1100 hours (CET) UNITED NATIONS CAUTION Reports published by the International Narcotics Control Board for 2020 TheReport of the International Narcotics Control Board for 2020 (E/INCB/2020/1) is supplemented by the following reports: Celebrating 60 Years of the Single Convention on Narcotic Drugs of 1961 and 50 Years of the Convention on Psychotropic Substances of 1971 (E/INCB/2020/1/Supp.1) Narcotic Drugs: Estimated World Requirements for 2021 — Statistics for 2019 (E/INCB/2020/2) Psychotropic Substances: Statistics for 2019 — Assessments of Annual Medical and Scientific Requirements for Substances in Schedules II, III and IV of the Convention on Psychotropic Sub- stances of 1971 (E/INCB/2020/3) Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psycho tropic Substances: Report of the International Narcotics Control Board for 2020 on the Implementation of Article 12 of the United Nations Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances of 1988 (E/INCB/2020/4) The updated lists of substances under international control, comprising narcotic drugs, psycho tropic substances and substances frequently used in the illicit manufacture of narcotic drugs and psychotropic substances, are contained in the latest editions of the annexes to the statistical forms (“Yellow List”, “Green List” and “Red List”), which are also issued by the Board. Contacting the International Narcotics Control Board The secretariat of the Board may be reached at the following address: Vienna International Centre Room E1339 P.O. -

Report on a Novel Emerging Class of Highly Potent Benzimidazole NPS Opioids: Chemical and in Vitro Functional Characterization of Isotonitazene
Received: 29 August 2019 Revised: 12 November 2019 Accepted: 13 November 2019 DOI: 10.1002/dta.2738 RESEARCH ARTICLE Report on a novel emerging class of highly potent benzimidazole NPS opioids: Chemical and in vitro functional characterization of isotonitazene Peter Blanckaert1† | Annelies Cannaert2† | Katleen Van Uytfanghe2 | Fabian Hulpia3 | Eric Deconinck4 | Serge Van Calenbergh3 | Christophe Stove2 1Scientific Direction Epidemiology and Public Health, Section Lifestyle and Chronic Diseases, Abstract Belgian Early Warning System Drugs This paper reports on the identification and full chemical characterization of (BEWSD), Sciensano, Brussels, Belgium isotonitazene (N,N-diethyl-2-[5-nitro-2-({4-[(propan-2-yl)oxy]phenyl}methyl)-1H-ben- 2Laboratory of Toxicology, Department of Bioanalysis, Faculty of Pharmaceutical zimidazol-1-yl]ethan-1-amine), a potent NPS opioid and the first member of the Sciences, Ghent University, Ghent, Belgium benzimidazole class of compounds to be available on online markets. Interestingly, 3Laboratory for Medicinal Chemistry, Department of Pharmaceutics, Faculty of this compound was sold under the name etonitazene, a structural analog. Identifica- Pharmaceutical Sciences, Ghent University, tion of isotonitazene was performed by gas chromatography mass spectrometry Ghent, Belgium (GC–MS) and liquid chromatography time-of-flight mass spectrometry (LC-QTOF- 4Scientific Direction Chemical and Physical Health Risks, Service of Medicines and Health MS), the latter identifying an exact-mass m/z value of 411.2398. All chromatographic Products, Sciensano, Brussels, Belgium data indicated the presence of a single, highly pure compound. Confirmation of the 1 13 Correspondence specific benzimidazole regio-isomer was performed using H and C NMR spectros- copy, after which the chemical characterization was finalized by recording Fourier- Christophe Stove, Laboratory of Toxicology, Department of Bioanalysis, Faculty of transform (FT-IR) spectra. -

Benzimidazole-Opioids June 2021
Drug Enforcement Administration Diversion Control Division Drug & Chemical Evaluation Section Benzimidazole-Opioids June 2021 Introduction: metonitazene, metodesnitazene, and protonitazene involved interaction with β-arrestin-2. Mu-opioid receptor and β-arrestin-2 Recently, several synthetic substances of benzimidazole interaction has been implicated in adverse health effects of many structural class are being trafficked and abused for their opioid- opioid analgesics. It is well established that mu-opioid receptor like effects. In the late 1950s, the pharmaceutical research agonists have a high potential for addiction and can produce dose- laboratories of the Swiss chemical company CIBA dependent respiratory depression and arrest. Abuse of these Aktiengesellschaft synthesized numerous benzimidazole- benzimidazole-opioids has led to their positive identification in opioids to include butonitazene, etodesnitazene, flunitazene, several toxicological cases in the United States. Specifically, metonitazene, metodesnitazene, and protonitazene. Since metonitazene has been identified in twenty post-mortem cases. 2019, the abuse of benzimidazole-opioids as evidenced by their identification in toxicology cases, similar to other synthetic opioids, has resulted in adverse health effects including deaths. User Population: As the United States continues to experience an unprecedented The population likely to abuse benzimidazole-opioids appears to be epidemic of opioid misuse and abuse, the continued evolution the same as those abusing prescription opioid analgesics, heroin, and increased trafficking and popularity of new and deadly and other synthetic opioid substances. This is evidenced by the synthetic opioids including benzimidazole-opioids with no types of other drugs co-identified in isotonitazene seizures and in approved medical use are of public health concern. fatal overdose cases. Toxicology analyses co-identified some of these benzimidazole-opioids with other opioids, stimulants, and Chemistry: benzodiazepines. -

Schedules of Controlled Substances (.Pdf)
PURSUANT TO THE TEXAS CONTROLLED SUBSTANCES ACT, HEALTH AND SAFETY CODE, CHAPTER 481, THESE SCHEDULES SUPERCEDE PREVIOUS SCHEDULES AND CONTAIN THE MOST CURRENT VERSION OF THE SCHEDULES OF ALL CONTROLLED SUBSTANCES FROM THE PREVIOUS SCHEDULES AND MODIFICATIONS. This annual publication of the Texas Schedules of Controlled Substances was signed by John Hellerstedt, M.D., Commissioner of Health, and will take effect 21 days following publication of this notice in the Texas Register. Changes to the schedules are designated by an asterisk (*). Additional information can be obtained by contacting the Department of State Health Services, Drugs and Medical Devices Unit, P.O. Box 149347, Austin, Texas 78714-9347. The telephone number is (512) 834-6755 and the website address is http://www.dshs.texas.gov/dmd. SCHEDULES Nomenclature: Controlled substances listed in these schedules are included by whatever official, common, usual, chemical, or trade name they may be designated. SCHEDULE I Schedule I consists of: -Schedule I opiates The following opiates, including their isomers, esters, ethers, salts, and salts of isomers, esters, and ethers, unless specifically excepted, if the existence of these isomers, esters, ethers, and salts are possible within the specific chemical designation: (1) Acetyl-α-methylfentanyl (N-[1-(1-methyl-2-phenethyl)-4-piperidinyl]-N- phenylacetamide); (2) Acetylmethadol; (3) Acetyl fentanyl (N-(1-phenethylpiperidin-4-yl)-N-phenylacetamide); (4) Acryl fentanyl (N-(1-phenethylpiperidin-4-yl)-N-phenylacrylamide) (Other name: -

Isotonitazene - Recently Identified in the Midwestern United States
November 2019 Potent Synthetic Opioid - Isotonitazene - Recently Identified in the Midwestern United States Purpose: The objective of this public announcement is to notify public health and public safety, law enforcement, clinicians, medical examiners and coroners, laboratory personnel, and all other related communities about new Demographics information surrounding the emergent synthetic opioid isotonitazene. Age: Background: Synthetic opioids are chemically manufactured drugs, often associated with unknown biological effects • Avg. 42, Med. 42.5 and health risks, a dangerous combination for any recreational drug user. Synthetic opioids are often prepared in • Range: 20’s to 60’s powder or tablet form and can be mixed with street level traditional opioids. In the United States, a staggering Sex: number of deaths have been reported in recent years linked to synthetic opioid use. The primary adverse effect most • Male (n=6), Female (n=2) commonly reported in association with synthetic opioid use is respiratory depression, often leading to death. Case Type: Summary: Isotonitazene is a potent synthetic opioid bearing structural resemblance to etonitazene, a synthetic opioid • Postmortem (n=8) that is nationally and internationally controlled. Isotonitazene is dissimilar in structure to popular synthetic opioids Specimen Type: typically encountered in forensic casework (e.g. fentanyl analogues, U-series analogues). Isotonitazene and similar • Blood (n=8) analogues (e.g. etonitazene, metonitazene, and clonitazene) were first synthesized and reported in the literature in the 1950s. Pharmacological data suggest that this group of synthetic opioids have potency similar to or greater than Date of Collection: fentanyl based on their structural modifications. Etonitazene is reported to be the most potent of the group followed • Aug. -

Ultra-High Performance Liquid Chromatography-High Resolution
International Journal of Molecular Sciences Article Ultra-High Performance Liquid Chromatography-High Resolution Mass Spectrometry and High-Sensitivity Gas Chromatography-Mass Spectrometry Screening of Classic Drugs and New Psychoactive Substances and Metabolites in Urine of Consumers Emilia Marchei 1,* , Maria Alias Ferri 2,3, Marta Torrens 2,3 , Magí Farré 3,4 , Roberta Pacifici 1, Simona Pichini 1 and Manuela Pellegrini 1 1 National Centre on Addiction and Doping, Istituto Superiore di Sanità, V.Le Regina Elena 299, 00161 Rome, Italy; roberta.pacifi[email protected] (R.P.); [email protected] (S.P.); [email protected] (M.P.) 2 Drug Addiction Program, Institut de Neuropsiquiatria i Addicions, Institut Hopsital del Mar d’Investigacions Mèdiques (INAD-IMIM), Parc de Salut Mar, 08003 Barcelona, Spain; [email protected] (M.A.F.); [email protected] (M.T.) 3 Department of Psychiatry and Pharmacology, Therapeutics and Toxicology, Universitat Autònoma de Barcelona, 08193 Cerdanyola del Vallés, Spain; [email protected] 4 Clinical Pharmacology Unit, Hospital Universitari Germans Trias i Pujol and Institut de Recerca Citation: Marchei, E.; Ferri, M.A.; GermansTrias i Pujol (HUGTiP-IGTP), 08916 Badalona, Spain Torrens, M.; Farré, M.; Pacifici, R.; * Correspondence: [email protected]; Tel.: +39-06-49903026 Pichini, S.; Pellegrini, M. Ultra-High Performance Liquid Abstract: The use of the new psychoactive substances is continuously growing and the implemen- Chromatography-High Resolution tation of accurate and sensible analysis -

Illinois Opioid Crisis Response Advisory Council Meeting February 8Th, 2021 MEETING MINUTES IDHS/SUPR Director David T
Illinois Opioid Crisis Response Advisory Council Meeting February 8th, 2021 MEETING MINUTES IDHS/SUPR Director David T. Jones welcomed the group. IDHS/SUPR Updates Director Jones gave the following updates: • The Illinois SUD Advisory Council met with SUPR to discuss holistic and integrative models of care for individuals with SUD. SUPR is particularly interested in mobile models of care to meet the unique needs of urban, suburban, and rural communities and connect people to treatment across the state. If you are interested in sharing ideas about mobile models please contact Ron Vlasaty, SUD Advisory Council Chairperson, at [email protected]. • On January 14th, HHS announced new practice guidelines for administering buprenorphine for treating OUD, including eliminating the X-waiver requirement. However, this announcement occurred during the transition between the Trump and Biden administrations, and the new guidelines will not be implemented at this time. The Biden administration stated that they are taking the guidelines under advisement and are committed to making buprenorphine more accessible to individuals with an OUD. • Discussion: o Council members inquired about SUD funds included in the COVID relief bill and the McKinsey settlement. SUPR is working with the Attorney General and IDPH to identify a process for how funds will be distributed. A recent report “Principles for the Use of Funds from the Opioid Litigation” released by Johns Hopkins University provides guidelines for spending and policies supported by the settlement funds. The report can be found at https://opioidprinciples.jhsph.edu/wp-content/uploads/2021/01/Litigation-Principles.pdf IDHS/Prescription Monitoring Program (PMP) Updates Sarah Pointer, PharmD, Clinical Director of the PMP, gave the following updates: • The PMP recently started the data sharing process with IDPH with the goal of displaying overdose data in PMP patient profiles. -

Volume 50 Number 42 Saturday, October 17, 2020 • Harrisburg, PA Pages 5721—5824
Volume 50 Number 42 Saturday, October 17, 2020 • Harrisburg, PA Pages 5721—5824 Agencies in this issue The General Assembly The Courts Department of Agriculture Department of Banking and Securities Department of Conservation and Natural Resources Department of Environmental Protection Department of Health Health Care Cost Containment Council Housing Finance Agency Insurance Department Legislative Reference Bureau Municipal Police Officers’ Education and Training Commission Pennsylvania Public Utility Commission Philadelphia Parking Authority State Board of Nursing State Real Estate Commission Susquehanna River Basin Commission Detailed list of contents appears inside. Latest Pennsylvania Code Reporter (Master Transmittal Sheet): Pennsylvania Bulletin Pennsylvania No. 551, October 2020 TYPE OR PRINT LEGIBLY Attn: 800 Church Rd. W. 17055-3198 PA Mechanicsburg, FRY COMMUNICATIONS, INC. COMMUNICATIONS, FRY CUT ON DOTTED LINES AND ENCLOSE IN AN ENVELOPE CHANGE NOTICE/NEW SUBSCRIPTION If information on mailing label is incorrect, please email changes to [email protected] or mail to: mail or [email protected] to changes email please incorrect, is label mailing on information If (City) (State) (Zip Code) label) mailing on name above number digit (6 NUMBER CUSTOMER NAME INDIVIDUAL OF NAME—TITLE OFFICE ADDRESS (Number and Street) (City) (State) (Zip The Pennsylvania Bulletin is published weekly by Fry PENNSYLVANIA BULLETIN Communications, Inc. for the Commonwealth of Pennsylva- nia, Legislative Reference Bureau, 641 Main Capitol Build- (ISSN 0162-2137) ing, Harrisburg, Pennsylvania 17120, under the policy supervision and direction of the Joint Committee on Docu- ments under 45 Pa.C.S. Part II (relating to publication and effectiveness of Commonwealth documents). The subscrip- tion rate is $87.00 per year, postpaid to points in the United States. -
![ADVANCED RELEASE EMCDDA Technical Report on the New Psychoactive Substance N,N- Diethyl-2-[[4-(1-Methylethoxy)Phenyl]Methyl]-5-N](https://docslib.b-cdn.net/cover/1311/advanced-release-emcdda-technical-report-on-the-new-psychoactive-substance-n-n-diethyl-2-4-1-methylethoxy-phenyl-methyl-5-n-2031311.webp)
ADVANCED RELEASE EMCDDA Technical Report on the New Psychoactive Substance N,N- Diethyl-2-[[4-(1-Methylethoxy)Phenyl]Methyl]-5-N
ADVANCED RELEASE EMCDDA technical report on the new psychoactive substance N,N- diethyl-2-[[4-(1-methylethoxy)phenyl]methyl]-5-nitro-1H- benzimidazole-1-ethanamine (isotonitazene) Explanatory note: In the interests of public health protection the EMCDDA is releasing this report before formal copy editing and page layout in the EMCDDA house style. The final report will be available on the EMCDDA website in due course. Authors: Michael Evans-Brown1, István Ujváry2, Joanna De Morais1, Rachel Christie1, Anabela Almeida1, Rita Jorge1, Ana Gallegos1, Roumen Sedefov1 1European Monitoring Centre for Drugs and Drug Addiction, Praça Europa 1, Cais do Sodré, 1249–289 Lisbon, Portugal 2iKem BT, Búza u. 32, Budapest 1033, Hungary Recommended citation: EMCDDA (2020), EMCDDA technical report on the new psychoactive substance N,N-diethyl-2-[[4-(1-methylethoxy)phenyl]methyl]-5-nitro-1H- benzimidazole-1-ethanamine (isotonitazene), EMCDDA, Lisbon. © European Monitoring Centre for Drugs and Drug Addiction, 2019 Praça Europa 1, Cais do Sodré, 1249–289 Lisbon, Portugal Tel: +351 211210200 Email: [email protected] Web: www.emcdda.europa.eu 1 Purpose The purpose of this technical report to provide an analyses of the available information on N,N-diethyl-2-[[4-(1-methylethoxy)phenyl]methyl]-5-nitro-1H-benzimidazole-1-ethanamine (commonly known as isotonitazene), an opioid analgesic that has recently emerged on the drug market in Europe, to support a risk assessment of the substance that has been requested by the European Commission in accordance with Article 5c of Regulation (EC) No 1920/2006 (as amended). Parts of this report were prepared under an EMCDDA contract (ref. -

Synthetic Opioids
Clinical Update: July 2021 NPS FOCUS: “NITAZENE” SYNTHETIC OPIOIDS Aegis launched an extensive and industry-leading testing menu of Novel Psychoactive Substances (NPS) in mid- 2020, which includes phenibut, tianeptine, xylazine, designer benzodiazepines, designer opioids, hallucinogens/dissociatives, synthetic cannabinoids, and synthetic stimulants, and continues to expand. In this month’s update, we will focus on the evolution of the non-fentanyl opioid analogue drug class commonly referred to as “nitazenes” and the challenges associated with detection and monitoring of these illicit substances. Origins of the “nitazenes” have been traced back to decades old pharmaceutical industry attempts to develop a novel opiate alternative to morphine.1-2 However, due to a high risk for abuse potential and reports of an overdose in early human studies, the “nitazenes” were deemed “not suitable for human consumption” and never gained approval for clinical use. With the increased regulation of older substances such as fentanyl and Schedule 1 illegal drugs, more varied chemical classes of opioids are in high demand. In response, the “nitazenes” have recently been “re-discovered” as an equally effective substitute and are illicitly marketed as a drug less detected in drug monitoring programs. Isotonitazene (also referred to as “iso”), is the prototypical, most frequently encountered member of the “nitazenes”. Iso is an extremely potent mu opioid receptor agonist and is structurally unrelated to fentanyl or traditional opioids.1 It first appeared in Canada and Europe in March 2019. By July 2019, isotonitazene was found in biological samples in the United States and has since been identified in over 250 drug overdose deaths. -
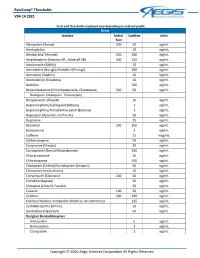
Paincomp® Thresholds V04.14.2021 Copyright © 2020 Aegis Sciences
PainComp® Thresholds V04.14.2021 Tests and thresholds employed vary depending on ordered profile Urine Analyte Initial Confirm Units Test Alprazolam (Xanax) 200 50 ng/mL Amitriptyline 10 ng/mL Amobarbital (Amytal) 200 200 ng/mL Amphetamine (Adzenys ER , Adderall XR) 500 250 ng/mL Aripiprazole (Abilify) 10 ng/mL Armodafinil (Nuvigil)/Modafinil (Provigil) 100 ng/mL Asenapine (Saphris) 10 ng/mL Atomoxetine (Strattera) 10 ng/mL Baclofen 500 ng/mL Benzodiazepines (Chlordiazepoxide, Clorazepate, 200 50 ng/mL Diazepam, Oxazepam, Temazepam) Brexpiprazole (Rexulti) 10 ng/mL Buprenorphine/Sublingual (Belbuca) 1 ng/mL Buprenorphine/Transdermal patch (Butrans) 1 ng/mL Bupropion (Aplenzin, Forfivo XL) 50 ng/mL Buspirone 25 ng/mL Butalbital 200 200 ng/mL Butorphanol 1 ng/mL Caffeine 15 mcg/mL Carbamazepine 50 ng/mL Cariprazine (Vraylar) 20 ng/mL Carisoprodol (Soma)/Meprobamate 200 ng/mL Chlorpromazine 10 ng/mL Chlorzoxazone 500 ng/mL Citalopram (Celexa)/Escitalopram (Lexapro) 50 ng/mL Clomipramine (Anafranil) 10 ng/mL Clonazepam (Klonopin) 200 50 ng/mL Clonidine (Kapvay) 50 ng/mL Clozapine (Clozaril, Fazaclo) 50 ng/mL Cocaine 100 50 ng/mL Codeine 200 100 ng/mL Cotinine/Nicotine metabolite (Habitrol, Nicoderm CQ) 125 ng/mL Cyclobenzaprine (Amrix) 10 ng/mL Dantrolene (Dantrium) 50 ng/mL Designer Benzodiazepines Adinazolam 5 ng/mL Bromazolam 1 ng/mL Clonazolam 1 ng/mL Copyright © 2020 Aegis Sciences Corporation All Rights Reserved PainComp® Thresholds V04.14.2021 Deschloroetizolam 1 ng/mL Diclazepam 1 ng/mL Etizolam 1 ng/mL Flualprazolam 1 ng/mL Flubromazepam -

Federal Register/Vol. 85, No. 118/Thursday, June 18, 2020
Federal Register / Vol. 85, No. 118 / Thursday, June 18, 2020 / Proposed Rules 36819 DEPARTMENT OF JUSTICE Legal Authority necessary to avoid an imminent hazard Section 201 of the CSA, 21 U.S.C. 811, to the public safety, the Administrator is Drug Enforcement Administration provides the Attorney General with the required to consider three of the eight authority to temporarily place a factors set forth in 21 U.S.C. 811(c): The 21 CFR Part 1308 substance in schedule I of the CSA for substance’s history and current pattern two years without regard to the of abuse; the scope, duration and [Docket No. DEA–631] requirements of 21 U.S.C. 811(b), if he significance of abuse; and what, if any, finds that such action is necessary to risk there is to the public health. 21 Schedules of Controlled Substances: avoid an imminent hazard to the public U.S.C. 811(h)(3). Consideration of these Temporary Placement of Isotonitazene safety. 21 U.S.C. 811(h)(1). In addition, factors includes actual abuse, diversion in Schedule I if proceedings to control a substance are from legitimate channels, and initiated under 21 U.S.C. 811(a)(1) while clandestine importation, manufacture, AGENCY: Drug Enforcement the substance is temporarily controlled or distribution. 21 U.S.C. 811(h)(3). Administration, Department of Justice. under section 811(h), the Attorney A substance meeting the statutory ACTION: Proposed amendment; notice of General may extend the temporary requirements for temporary scheduling intent. scheduling for up to one year. 21 U.S.C.