Complex Balanced Chromosomal Translocation T(2;5;13) (P21;P15;Q22
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Whole Chromosome Gain Does Not in Itself Confer Cancer-Like Chromosomal Instability
Whole chromosome gain does not in itself confer cancer-like chromosomal instability Anders Valinda,1, Yuesheng Jina, Bo Baldetorpb, and David Gisselssona aDepartment of Clinical Genetics, Lund University, University and Regional Laboratories, Biomedical Center B13, Lund SE22184, Sweden; and bDepartment of Oncology, Lund University, Skåne University Hospital, Lund SE22185, Sweden Edited* by George Klein, Karolinska Institutet, Stockholm, Sweden, and approved November 4, 2013 (received for review June 12, 2013) Constitutional aneuploidy is typically caused by a single-event and chromosomal instability in humans is using constitutional meiotic or early mitotic error. In contrast, somatic aneuploidy, aneuploidy syndromes as a model. Cells from patients with these found mainly in neoplastic tissue, is attributed to continuous syndromes provide a good experimental system for studying the chromosomal instability. More debated as a cause of aneuploidy effects of aneuploidy on overall genome stability on representative is aneuploidy itself; that is, whether aneuploidy per se causes human material. Such cells typically only have a single or a limited chromosomal instability, for example, in patients with inborn set of stem-line chromosome aberrations compared with tumor aneuploidy. We have addressed this issue by quantifying the level cell lines, which typically harbor a multitude of genetic lesions, as of somatic mosaicism, a proxy marker of chromosomal instability, well as a cancer phenotype. The few earlier studies performed on in patients with -

Cytogenetics, Chromosomal Genetics
Cytogenetics Chromosomal Genetics Sophie Dahoun Service de Génétique Médicale, HUG Geneva, Switzerland [email protected] Training Course in Sexual and Reproductive Health Research Geneva 2010 Cytogenetics is the branch of genetics that correlates the structure, number, and behaviour of chromosomes with heredity and diseases Conventional cytogenetics Molecular cytogenetics Molecular Biology I. Karyotype Definition Chromosomal Banding Resolution limits Nomenclature The metaphasic chromosome telomeres p arm q arm G-banded Human Karyotype Tjio & Levan 1956 Karyotype: The characterization of the chromosomal complement of an individual's cell, including number, form, and size of the chromosomes. A photomicrograph of chromosomes arranged according to a standard classification. A chromosome banding pattern is comprised of alternating light and dark stripes, or bands, that appear along its length after being stained with a dye. A unique banding pattern is used to identify each chromosome Chromosome banding techniques and staining Giemsa has become the most commonly used stain in cytogenetic analysis. Most G-banding techniques require pretreating the chromosomes with a proteolytic enzyme such as trypsin. G- banding preferentially stains the regions of DNA that are rich in adenine and thymine. R-banding involves pretreating cells with a hot salt solution that denatures DNA that is rich in adenine and thymine. The chromosomes are then stained with Giemsa. C-banding stains areas of heterochromatin, which are tightly packed and contain -

Double Aneuploidy in Down Syndrome
Chapter 6 Double Aneuploidy in Down Syndrome Fatma Soylemez Additional information is available at the end of the chapter http://dx.doi.org/10.5772/60438 Abstract Aneuploidy is the second most important category of chromosome mutations relat‐ ing to abnormal chromosome number. It generally arises by nondisjunction at ei‐ ther the first or second meiotic division. However, the existence of two chromosomal abnormalities involving both autosomal and sex chromosomes in the same individual is relatively a rare phenomenon. The underlying mechanism in‐ volved in the formation of double aneuploidy is not well understood. Parental ori‐ gin is studied only in a small number of cases and both nondisjunctions occurring in a single parent is an extremely rare event. This chapter reviews the characteristics of double aneuploidies in Down syndrome have been discussed in the light of the published reports. Keywords: Double aneuploidy, Down Syndrome, Klinefelter Syndrome, Chromo‐ some abnormalities 1. Introduction With the discovery in 1956 that the correct chromosome number in humans is 46, the new area of clinical cytogenetic began its rapid growth. Several major chromosomal syndromes with altered numbers of chromosomes were reported, such as Down syndrome (trisomy 21), Turner syndrome (45,X) and Klinefelter syndrome (47,XXY). Since then it has been well established that chromosome abnormalities contribute significantly to genetic disease resulting in reproductive loss, infertility, stillbirths, congenital anomalies, abnormal sexual development, mental retardation and pathogenesis of malignancy [1]. Clinical features of patients with common autosomal or sex chromosome aneuploidy is shown in Table 1. © 2015 The Author(s). Licensee InTech. This chapter is distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. -

The Combined Test
Antenatal Screening Antenatal Screening for Down's syndrome, Edwards syndrome and Patau syndrome The Combined Test Questions and Answers for women considering the test The Wolfson Institute of Preventive Medicine Barts and The London School of Medicine and Dentistry Antenatal Screening This leaflet answers some of the common questions women ask about their screening test – we hope you find it helpful. You are welcome to discuss the test with your midwife or consultant before you decide whether you would like to be screened. If you have any further questions screening staff at the Wolfson Institute are available to talk to you on 020 7882 6293. What is Down's syndrome? What are Edwards and Patau syndrome? Down's syndrome (trisomy 21) is Edwards syndrome (trisomy 18) is defined by the presence of an extra defined by the presence of an extra chromosome number 21 in the cells chromosome number 18 in the cells of the fetus or affected individual. In of the fetus or affected individual an unscreened population about 1 in while Patau syndrome (trisomy 13) every 500 babies is born with Down's is defined by an extra chromosome syndrome. Usually it is not inherited number 13 in the cells of the fetus or and so a baby can be affected affected individual. Both syndromes even if there is no history of Down's affect multiple organs with a high risk syndrome in the family. of fetal death. Down's syndrome is the most At 12 weeks of pregnancy Edwards common cause of severe learning syndrome has a prevalence of about disability and is often associated 1 in 1,500 and Patau syndrome has a with physical problems such as heart prevalence of about 1 in 3,500 in an defects and difficulties with sight and unscreened population. -

Congenital Heart Disease and Chromossomopathies Detected By
Review Article DOI: 10.1590/0103-0582201432213213 Congenital heart disease and chromossomopathies detected by the karyotype Cardiopatias congênitas e cromossomopatias detectadas por meio do cariótipo Cardiopatías congénitas y anomalías cromosómicas detectadas mediante cariotipo Patrícia Trevisan1, Rafael Fabiano M. Rosa2, Dayane Bohn Koshiyama1, Tatiana Diehl Zen1, Giorgio Adriano Paskulin1, Paulo Ricardo G. Zen1 ABSTRACT Conclusions: Despite all the progress made in recent de- cades in the field of cytogenetic, the karyotype remains an es- Objective: To review the relationship between congenital sential tool in order to evaluate patients with congenital heart heart defects and chromosomal abnormalities detected by disease. The detailed dysmorphological physical examination the karyotype. is of great importance to indicate the need of a karyotype. Data sources: Scientific articles were searched in MED- LINE database, using the descriptors “karyotype” OR Key-words: heart defects, congenital; karyotype; Down “chromosomal” OR “chromosome” AND “heart defects, syndrome; trisomy; chromosome aberrations. congenital”. The research was limited to articles published in English from 1980 on. RESUMO Data synthesis: Congenital heart disease is characterized by an etiologically heterogeneous and not well understood Objetivo: Realizar uma revisão da literatura sobre a group of lesions. Several researchers have evaluated the pres- relação das cardiopatias congênitas com anormalidades ence of chromosomal abnormalities detected by the karyo- cromossômicas detectadas por meio do exame de cariótipo. type in patients with congenital heart disease. However, Fontes de dados: Pesquisaram-se artigos científicos no most of the articles were retrospective studies developed in portal MEDLINE, utilizando-se os descritores “karyotype” Europe and only some of the studied patients had a karyo- OR “chromosomal” OR “chromosome” AND “heart defects, type exam. -

Soft UK Your Unborn Baby A5 04.10 37045
SOFT UK Support Organisation for Trisomy 13/18 and related disorders Charity number 1002918 www.soft.org.uk Your Unborn Baby SOFT provides support for families affected by Patau’s syndrome (trisomy 13), Edwards’ syndrome (trisomy 18), partial trisomy, mosaicism, rings, translocation, deletion and related abnormalities and rare conditions including holoprosencephaly. Truth has no special time of its own. Its hour is now. Always. Albert Schweitzer YOUR UNBORN BABY INDEX Section Page About Your Unborn Baby 2 SOFT UK Booklets 4 For Professionals 4 Chromosome Defects, What are They? 5 Trisomy 7 Holoprosencephaly 8 Related Disorders 9 Genetic Information 10 Screening Tests 13 Diagnostic tests 15 Telling Parents 18 Making a Decision 19 When the Pregnancy is Terminated 22 When the Pregnancy Continues 26 Caring for a Baby with a Related Disorder 32 Bereavement 34 How Brothers and Sisters are Affected 39 A New Baby 43 Acknowledgments 45 SOFT UK 45 Information for Parents 45 Other Sources of Support for Parents 47 1 ABOUT YOUR UNBORN BABY Your Unborn Baby was compiled by Rachel Attwell, Jenny Robbins, and Christine Rose, with the help of SOFT medical advisers and families affected by trisomy 13/18 who have shared their own personal experiences. Every family is unique. What may be right for one child or family is not necessarily right for another, and parents can use the shared memories in these booklets as a basis for discussion between themselves and their medical advisers. SOFT does not recommend particular methods of treatment, and new treatment must never be started or existing treatment changed without consultation with your doctor and other medical professionals. -

A Newborn with Trisomy 13 Presenting with Cloacal Exstrophy
The Turkish Journal of Pediatrics 2015; 57: 198-201 Case Report A newborn with trisomy 13 presenting with cloacal exstrophy Şebnem Kader1, Mehmet Mutlu1, Mehmet Sarıaydın1, Yakup Aslan1, Erol Erduran2, Alper Han Çebi3 Divisions of 1Neonatology and 2Pediatric Hematology, Department of Pediatrics, and 3Department of Medical Genetics, Karadeniz Technical University Faculty of Medicine, Trabzon, Turkey E-mail: [email protected] Received: 20 June 2014, Revised: 28 August 2014, Accepted: 26 September 2014 SUMMARY : Kader Ş, Mutlu M, Sarıaydın M, Aslan Y, Erduran E, Çebi A. A newborn with trisomy 13 presenting with cloacal exstrophy. Turk J Pediatr 2015; 57: 198-201. Trisomy 13 syndrome is a rare disorder that carries a high mortality rate due to abnormal prenatal development resulting in serious birth defects. Although genitourinary malformations are commonly seen in trisomy 13 syndrome, to our knowledge, the association of cloacal exstrophy with trisomy 13 has been extremely rarely reported. Herein, a newborn with trisomy 13 syndrome having multiple congenital anomalies, including cloacal exstrophy, is presented, and the importance of structural anomalies of the neutrophilic leukocytes on a blood smear in supporting diagnosis of trisomy 13 is discussed. Key words: trisomy 13 syndrome, cloacal exstrophy, neutrophilic leukocytes, nuclear projections, blood smear, newborn. Trisomy 13, also called Patau syndrome, is care during her pregnancy. The mother’s third a rare genetic disorder that is characterized pregnancy had been terminated due to molar by severe to profound intellectual disability pregnancy. There was no consanguinity. The and multiple congenital anomalies, including family history revealed no birth defects or orofacial clefts, microphthalmia or anophthalmia, congenital anomalies. -
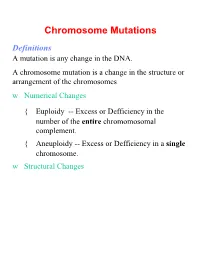
Chromosome Mutations Definitions a Mutation Is Any Change in the DNA
Chromosome Mutations Definitions A mutation is any change in the DNA. A chromosome mutation is a change in the structure or arrangement of the chromosomes w Numerical Changes { Euploidy -- Excess or Defficiency in the number of the entire chromomosomal complement. { Aneuploidy -- Excess or Defficiency in a single chromosome. w Structural Changes Numerical Chromosome Mutations Euploid -- “True Number” The cell(s) have the same number of copies of all the chromosome. 1. Monoploid (Haploid if a gamete) 2. Diploid (Often is not considered a Euploid) 3. Triploid 4. Tetraploid Euploidy Chromosomes shown at metaphase Normal Diploid Euploid Monoploid Triploid Tetraploid Euploidy is an excess or deficiency for the entire complement of chromosomes. Euploidy Organisms w Plants, amphibians, lower reptiles w Some cells in other organisms (liver, midgut, etc.) Root Causes w Nondisjunction w Autoreduplication Euploidy (continued) Origins w Autopolyploidy { Often involving individuals within the same species. { Infertile. Propagation by cloning. w Allopolyploidy { Often involving individuals from different species. { Fertile. Dosage Concerns Examples Numerical Chromosome Mutations Aneuploidy Excess or Defficiency in a single chromosome. 1. Monosomy 2. Disomy (only if normally cell is not diploid) 3. Trisomy 4. Tetrasomy Aneuploidy Chromosomes shown at metaphase Normal Diploid Aneuploid Monosomic Trisomic Tetrasomic Aneuploidy is an excess or deficiency for fewer than the entire complement of chromosomes. Aneuploidy Organisms w All organisms w Variability in how they deal with it Root Causes w Nondisjunction w Results of Chromosome Structure Mutations Aneuploidy -- Examples Plants w Jimson Weed (Datura stramonium) Animals w Drosophila: Triploid females are viable & fertile. Trisomy flies are not! w Human (autosomal): { Trisomy 13 (Patau Syndrome) 1:20,000 { Trisomy 18 (Edwards Syndrome) 1:10,000 { Trisomy 21 (Down Syndrome) 1:700 { Other rare ones (7, 9, 12, 14, & 22) Aneuploidy Examples w Human Sex Determining Chromosomes 1. -

What Do We Know Now About Trisomy 13?
The Support Organization for Trisomy 18, 13 and Related Disorders What Do We Know Now About Trisomy 13? Trisomy 13 syndrome (Patau syndrome) is a disorder of human chromosomes which occurs in approximately 1 in 10,000-25,000 live-born infants. Trisomy refers to three copies of a chromosome instead of the normal two and in Trisomy 13 there is the presence of an extra #13 chromosome. Approximately 80% of infants with Trisomy 13 syndrome will have a full trisomy (affecting all cells) while the remainder will have a trisomy due to a rearrangement of cells called a translocation (an attachment of all or part of one chromosome to another chromosome) or have mosaicism (two different cell lines in an individual). Most often a diagnosis of Trisomy 13 is suspected by findings seen The new non-invasive prenatal test (NIPT) is on fetal ultrasound, or screening by maternal blood tests. Optional increasing in use. A Positive Predictive invasive testing, amniocentesis or chorionic villus sampling, is Value (PPV) calculator is a tool used to needed to confirm a diagnosis but carry a small risk to the fetus. A prenatal diagnosis of Trisomy 13, before 24 weeks, is often determine accuracy of a NIPT positive result. followed with an option to terminate; a decision made by 75% in the USA and 90% in Europe. A diagnosis can affect the care provided to those continuing pregnancy, their birthing options, and the care of a liveborn infant. Infants born with Trisomy 13 have a recognizable pattern of physical features that often allows the health professional to make the diagnosis of the syndrome. -

Prenatal Genetic Screening
PRENATAL GENETIC SCREENING Background 1. Definition of prenatal genetic screening o Tests done to determine if unborn child at risk for genetic disorder 2. Testing types1 o Noninvasive/mildly invasive . Ultrasound . Maternal serum testing o Invasive . Chorionic villus sampling . Amniocentesis . Percutaneous umbilical cord sampling Pathophysiology 1. Pathology of Disease o Aneuploidy2 . Klinefelter syndrome . Trisomy 13 (Patau syndrome) . Trisomy 18 (Edward’s syndrome) . Trisomy 21 (Down’s syndrome) . Turner’s syndrome o Autosomal dominant . Huntington’s chorea o Autosomal recessive2 . Cystic fibrosis . Alpha-thalassemia . Beta-thalassemia . Familial dysautonomia . Phenylketonuria (PKU) . Sickle cell anemia . Tay-Sachs disease o Polygenic2 . Cleft faces (lips, palate) . Neural tube defects o X-Linked dominant . Fragile X syndrome o X-Linked recessive . Duchenne’s muscular dystrophy . Hemophilia A o Spontaneous mutation 2. Incidence2 o Alpha-thalassemia- 1:2500 in Southeast Asian descent o Beta-thalassemia- 1:2500 in Greek/Italian descent o Cleft faces (lips, palate)- 1/100 to 1/20 with an affected parent or after having one previous affected child Prenatal Genetic Screening Page 1 of 6 9.6.12 o Cystic fibrosis- 1:2500 in whites of European or Ashkenazi Jewish descent o Duchenne’s muscular dystrophy- 1:3600-6000 live male births o Familial dysautonomia- 1:2500 in Ashkenazi Jewish descent o Fragile X syndrome- 1:4000 live male births, 1:6000-8000 live female births o Hemophilia A- 1:5000 live male births o Huntington’s chorea- incidence not -

Medical Diagnosis/Conditions for Eligibility in AEIS
Medical Diagnosis/Conditions for Eligibility in AEIS 1) Achondroplasia 2) Agenesis of Corpus Callosum 3) Agyria (Lissencephaly) 4) Albinism 5) Amniotic Band syndrome 6) Anencephaly 7) Angelman’s syndrome 8) Anophthalmia 9) Apert syndrome 10) Aplasia of the brain (brain malformation/abnormality) 11) Arhinencephaly (Holoprosencephaly) 12) Arnold-Chiari syndrome 13) Arthrogryposis 14) Asperger syndrome/disorder 15) Asphyxiating Thoracic Dystrophy (Jeune syndrome) 16) Attachment disorder 17) Autism/Autism Spectrum disorder 18) Bardet-Biedl syndrome 19) Brain injury/degeneration 20) Brain malformation/abnormality 21) Cerebral Palsy (all types) 22) CHARGE syndrome 23) Chiari Malformation 24) Childhood Depression 25) Childhood Disintegrative disorder 26) Cornelia de Lange syndrome 27) Cortical vision impairment (vision loss/impairment) 28) Cri-du-Chat syndrome 29) Cytomegalovirus (CMV) 30) Dandy Walker syndrome/variant 31) De Morsier syndrome (Septo-Optic Dysplasia) 32) Developmental Apraxia 33) DiGeorge syndrome 34) Dilantin syndrome (Fetal Hydantoin syndrome) 35) Down Syndrome (Trisomy 21) 36) Edwards syndrome (Trisomy 18) 37) Encephalomalacia 38) Encephalopathy 39) Epilepsy (seizure disorder) 40) Fetal Alcohol syndrome 41) Fetal Hydantoin syndrome (Dilantin syndrome) 42) Fragile X syndrome 43) Genetic/Chromosomal malformation/abnormality (not listed) 44) Hearing Loss/Impairment 45) Heart Disease/Defect (not listed) 46) Hemiplegia 47) Herpes Simplex Virus (HSV) 48) Holoprosencephaly (Arhinencephaly) 49) Holt Oram syndrome 50) Hydraencephaly -

Chromosomal Study in Newborn Infants with Congenital Anomalies in Assiut University Hospital: Cross-Sectional Study
The Egyptian Journal of Medical Human Genetics (2011) 12, 79–90 Ain Shams University The Egyptian Journal of Medical Human Genetics www.ejmhg.eg.net www.sciencedirect.com ORIGINAL ARTICLE Chromosomal study in newborn infants with congenital anomalies in Assiut University hospital: Cross-sectional study Yasir A. Mohammed a,*, Rabah M. Shawky b, Amal A.S. Soliman c, Maher M. Ahmed c a Tema Hospital, Ministry of Health, Sohag, Egypt b Pediatric Department, Ain Shams University, Cairo, Egypt c Pediatric Department, Assiut University, Assiut, Egypt Received 8 August 2010; accepted 8 January 2011 KEYWORDS Abstract In 40–60% of congenital malformations, the cause is unknown. Genetic factors account Chromosomes; for approximately 15%; environmental factors produce approximately 10%; a combination of Congenital anomalies; genetic and environmental influences produces 20–25%. The study aims to document prevalence Karyotype; and patterns of congenital malformations detected at birth in Assiut University hospital and clarify Malformations; underlying chromosomal abnormalities of such malformations. Also possible predisposing factors Egypt will be studied. Newborns with apparent congenital anomalies were selected from 5000 newborn infants delivered consecutively at the department of Obstetrics and Gynecology within 7 months. Full maternal his- tory, family history, perinatal history, pedigree construction as well as clinical examinations and investigations including karyotype were done. Congenital anomalies were found in 103 cases with a prevalence of 2.06% with male to female ratio of 1.7:1. Skeletal system anomalies had the highest * Corresponding author. Address: Sohag Governorate, Al Gomhoria Street, Egypt. Tel.: +022 0105339867/93789863. E-mail addresses: [email protected] (Y.A. Mohammed), shawkyr- [email protected] (R.M.