Instruction Booklet for Engineering
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

14/2010/L1 SUBJECT NO. 119 Sub : STATUS OF
RC.No: 14/2010/L1 SUBJECT NO. 119 Sub : STATUS OF DEVELOPMENT PLAN TRANSPOSRT CORRIDOR FOR VUDA AREAS- Reg. *** AGENDA NOTE : It is submitted that the Govt vide GO.Ms.No: 616 MA, dt: 16.10.2009 has constituted unified metropolitan transport committee (UMTC) under the Chairmanship of Principal Secretary to Govt MA&UD Dept for effective implementation and coordination of the various traffic and transportation measures. As soon as the Govt has issued above GO, the Commissioner, GVMC Commissioners of Municipalities in VMR and the officials of other departments like Railways, DT & CP, RTC, R&B etc., are addressed vide this office letter dt: 29.01.2010 and requested to furnish the relevant issues pertaining to traffic and transportation initiating if any in their respective areas so as to enable to prepare comprehensive proposal to place the same before the committee. Further the Principal Secretary to Govt MA&UD Dept vide this office letter dt: 8.01.2012 was also requested to address the Ministry of Urban Development, Govt of India to accord sanction for comprehensive traffic and transportation study for VMR on which the Principal Secretary to Govt, MA & UD, vide letter dt: 6.02.2012 is pleased to address the Secretary, Ministry of Urban Development Govt of India to accord sanction for comprehensive traffic and transportation study for VMR under the National urban Transport policy and also grant financial assistance. Also submitted that a meeting was conducted by VUDA on Unified Metropolitan Traffic and Transportation with the Dist Collector, Visakhapatnam, Commissioner of Police, Visakhapatnam city, Superintendent of Police, Visakhapatnam (Rural) GVMC officials etc., and a PowerPoint presentation was also given on the traffic and transportation issues on 25.6.2013. -

FINAL RFP of Museum at Beach Latest.Pdf
RFP Visakhapatnam Urban Development Authority. 13th July’ 2018 RFP for “Selection of Architectural and Design Consultant for Setting up of an Integrated Naval Museum along with Sea Harrier, Parking and Landscaping in Beach Road in Visakhapatnam.” ISSUED BY: VISAKHAPATNAM URBAN DEVELOPMENT AUTHORITY ANDHRA PRADESH INDIA “Selection of Architectural and Design Consultant for Setting up of an Integrated Naval Museum along with Sea Harrier, Parking and Landscaping in Beach Road in Visakhapatnam.” 1. DISCLAIMER The information contained in the Request for Proposal document (“RFP”) is provided to Bidders on the terms and conditions set out in this RFP and such other terms and conditions subject to which such information is provided. The RFP is not an agreement and is neither an offer nor invitation by the Authority to the prospective Bidders or any other person. The purpose of the RFP is to provide interested parties with information that may be useful to them in the formulation of their Proposals pursuant to this RFP. The RFP includes statements, which reflect various assumptions and assessments arrived at by the Authority in relation to the Consultancy. Such assumptions, assessments and statements do not purport to contain all the information that each Bidder may require. The RFP may not be appropriate for all persons, and it is not possible for the Authority, its employees or advisers to consider the objectives, technical expertise and particular needs of each party who reads or uses the RFP. The assumptions, assessments, statements and information contained in the RFP, may not be complete, accurate, adequate or correct. Each Bidder should, therefore, conduct its own investigations and analysis and should check the accuracy, adequacy, correctness, reliability and completeness of the assumptions, assessments and information contained in the RFP and obtain independent advice from appropriate sources. -

Volume9 Issue11(6)
Volume 9, Issue 11(6), November 2020 International Journal of Multidisciplinary Educational Research Published by Sucharitha Publications Visakhapatnam Andhra Pradesh – India Email: [email protected] Website: www.ijmer.in Editorial Board Editor-in-Chief Dr.K. Victor Babu Associate Professor, Institute of Education Mettu University, Metu, Ethiopia EDITORIAL BOARD MEMBERS Prof. S. Mahendra Dev Prof. Igor Kondrashin Vice Chancellor The Member of The Russian Philosophical Indira Gandhi Institute of Development Society Research, Mumbai The Russian Humanist Society and Expert of The UNESCO, Moscow, Russia Prof.Y.C. Simhadri Vice Chancellor, Patna University Dr. Zoran Vujisiæ Former Director Rector Institute of Constitutional and Parliamentary St. Gregory Nazianzen Orthodox Institute Studies, New Delhi & Universidad Rural de Guatemala, GT, U.S.A Formerly Vice Chancellor of Benaras Hindu University, Andhra University Nagarjuna University, Patna University Prof.U.Shameem Department of Zoology Prof. (Dr.) Sohan Raj Tater Andhra University Visakhapatnam Former Vice Chancellor Singhania University, Rajasthan Dr. N.V.S.Suryanarayana Dept. of Education, A.U. Campus Prof.R.Siva Prasadh Vizianagaram IASE Andhra University - Visakhapatnam Dr. Kameswara Sharma YVR Asst. Professor Dr.V.Venkateswarlu Dept. of Zoology Assistant Professor Sri.Venkateswara College, Delhi University, Dept. of Sociology & Social Work Delhi Acharya Nagarjuna University, Guntur I Ketut Donder Prof. P.D.Satya Paul Depasar State Institute of Hindu Dharma Department of Anthropology Indonesia Andhra University – Visakhapatnam Prof. Roger Wiemers Prof. Josef HÖCHTL Professor of Education Department of Political Economy Lipscomb University, Nashville, USA University of Vienna, Vienna & Ex. Member of the Austrian Parliament Dr.Kattagani Ravinder Austria Lecturer in Political Science Govt. Degree College Prof. -
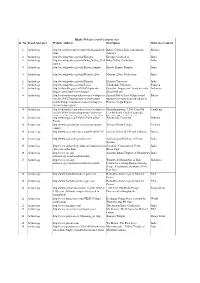
2.Hindu Websites Sorted Category Wise
Hindu Websites sorted Category wise Sl. No. Broad catergory Website Address Description Reference Country 1 Archaelogy http://aryaculture.tripod.com/vedicdharma/id10. India's Cultural Link with Ancient Mexico html America 2 Archaelogy http://en.wikipedia.org/wiki/Harappa Harappa Civilisation India 3 Archaelogy http://en.wikipedia.org/wiki/Indus_Valley_Civil Indus Valley Civilisation India ization 4 Archaelogy http://en.wikipedia.org/wiki/Kiradu_temples Kiradu Barmer Temples India 5 Archaelogy http://en.wikipedia.org/wiki/Mohenjo_Daro Mohenjo_Daro Civilisation India 6 Archaelogy http://en.wikipedia.org/wiki/Nalanda Nalanda University India 7 Archaelogy http://en.wikipedia.org/wiki/Taxila Takshashila University Pakistan 8 Archaelogy http://selians.blogspot.in/2010/01/ganesha- Ganesha, ‘lingga yoni’ found at newly Indonesia lingga-yoni-found-at-newly.html discovered site 9 Archaelogy http://vedicarcheologicaldiscoveries.wordpress.c Ancient Idol of Lord Vishnu found Russia om/2012/05/27/ancient-idol-of-lord-vishnu- during excavation in an old village in found-during-excavation-in-an-old-village-in- Russia’s Volga Region russias-volga-region/ 10 Archaelogy http://vedicarcheologicaldiscoveries.wordpress.c Mahendraparvata, 1,200-Year-Old Cambodia om/2013/06/15/mahendraparvata-1200-year- Lost Medieval City In Cambodia, old-lost-medieval-city-in-cambodia-unearthed- Unearthed By Archaeologists 11 Archaelogy http://wikimapia.org/7359843/Takshashila- Takshashila University Pakistan Taxila 12 Archaelogy http://www.agamahindu.com/vietnam-hindu- Vietnam -

Jagan Govt Makes Employment History
Follow us on: RNI No. APENG/2018/764698 @TheDailyPioneer facebook.com/dailypioneer Established 1864 Published From ANALYSIS 7 CELEB TALKS 10 SPORTS 12 VIJAYAWADA DELHI LUCKNOW BHOPAL GANDHIGIRI MARDAANI 2 WILL SEE WOMEN CAN’T TAKE PROTEAS RAIPUR CHANDIGARH BHUBANESWAR IN REAL LIFE STAND UP AGAINST EVIL: RANI LIGHTLY: RAHANE RANCHI DEHRADUN HYDERABAD *Late City Vol. 1 Issue 336 VIJAYAWADA, TUESDAY OCTOBER 1, 2019; PAGES 12 `3 *Air Surcharge Extra if Applicable FELT THE PRESSURE OF DOING A FEMALE- CENTRIC FILM { Page 11 } www.dailypioneer.com All is well SEEKING DIVINE BLESSINGS with NMDC JAGAN GOVT MAKES sourcing, asserts RINL VISAKHAPATNAM: RINL, EMPLOYMENT HISTORY the corporate entity of Visakhapatnam Steel Plant strongly refuted the allega- l Appoints 1.26 lakh persons in one recruitment drive tions made by CPI leader JV Satyanarayana Murthy that PNS n AMARAVATI the management of RINL picked up a confrontation Andhra Pradesh government with NMDC. on Monday appointed over RINL management termed 1.26 lakh employees, said to be the allegations as "baseless the first of its kind in the and lacks substance". It is country in terms of numbers in clarified that RINL and a single recruitment drive, NMDC have been maintain- under a new governance initia- ing excellent relationship with tive of Village and Ward regard to supply of iron ore. Secretariats, being launched "At time, supply of iron ore on October 2. YS Jaganmohan Reddy presenting ‘pattu vastralu’ to Lord Venkateswara at tirumala on Monday from NMDC mines in "This should be written in Chhattisgarh gets disrupted golden letters in the country's during the breakdown of KK history. -
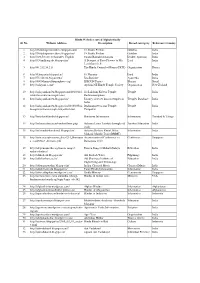
Hindu Websites Sorted Alphabetically Sl
Hindu Websites sorted Alphabetically Sl. No. Website Address Description Broad catergory Reference Country 1 http://18shaktipeetasofdevi.blogspot.com/ 18 Shakti Peethas Goddess India 2 http://18shaktipeetasofdevi.blogspot.in/ 18 Shakti Peethas Goddess India 3 http://199.59.148.11/Gurudev_English Swami Ramakrishnanada Leader- Spiritual India 4 http://330milliongods.blogspot.in/ A Bouquet of Rose Flowers to My Lord India Lord Ganesh Ji 5 http://41.212.34.21/ The Hindu Council of Kenya (HCK) Organisation Kenya 6 http://63nayanar.blogspot.in/ 63 Nayanar Lord India 7 http://75.126.84.8/ayurveda/ Jiva Institute Ayurveda India 8 http://8000drumsoftheprophecy.org/ ISKCON Payers Bhajan Brazil 9 http://aalayam.co.nz/ Ayalam NZ Hindu Temple Society Organisation New Zealand 10 http://aalayamkanden.blogspot.com/2010/11/s Sri Lakshmi Kubera Temple, Temple India ri-lakshmi-kubera-temple.html Rathinamangalam 11 http://aalayamkanden.blogspot.in/ Journey of lesser known temples in Temples Database India India 12 http://aalayamkanden.blogspot.in/2010/10/bra Brahmapureeswarar Temple, Temple India hmapureeswarar-temple-tirupattur.html Tirupattur 13 http://accidentalhindu.blogspot.in/ Hinduism Information Information Trinidad & Tobago 14 http://acharya.iitm.ac.in/sanskrit/tutor.php Acharya Learn Sanskrit through self Sanskrit Education India study 15 http://acharyakishorekunal.blogspot.in/ Acharya Kishore Kunal, Bihar Information India Mahavir Mandir Trust (BMMT) 16 http://acm.org.sg/resource_docs/214_Ramayan An international Conference on Conference Singapore -

6. Oppositional Noise from the Fringes
6. Oppositional Noise from the Fringes Despite the pessimistic findings of the previous chapter, where government mediation overall showed little concern for upholding the social and environmental legislation intended to protect Adivasis, there were many actors who worked towards a broader and more inclusive perspective. Over the years, a large number of individuals and organisations have protested against bauxite mining plans on the grounds of a need to protect Adivasi land and the impossibility of properly compensating those who would be affected. For opposition to the Jindal South West (JSW) bauxite project to have a significant impact, it would have to open the government to engagement outside of the private sphere of policymaking and project implementation where issues were being addressed with select business interests. Meaningful mediation depends not so much on government capacity but on the amount of pressure civil society actors are able to exert. The first challenge for opponents of the JSW bauxite project was to find enough grassroots support to allow for large-scale mobilisation at the proposed project sites. If large numbers turned out, this would give increased legitimacy to oppositional activities, but given their severe lack of resources, poor people in the remote hills of the Agency would find it very difficult to create and sustain an opposition movement. Nevertheless, tribal social movements have at times been very strong in Andhra Pradesh as well as in many other parts of India. For example, local people protested strongly against the proposed bauxite project in Kashipur, in the neighbouring state of Odisha. As one activist commented: 133 Landlock Kakinada is the one place in Andhra Pradesh where there is a strong local movement against a project. -

Figure to Be Inserted
Research Report 10 Figure to be inserted Cities versus Agriculture: Revisiting Intersectoral Water Transfers, Potential Gains and Conflicts François Molle and Jeremy Berkoff Postal Address: IWMI, P O Box 2075, Colombo, Sri Lanka Location: 127 Sunil Mawatha, Pelawatte, Battaramulla, Sri Lanka International Telephone: +94-11 2787404, 2784080 Fax: +94-11 2786854 Water Management Email: [email protected] Website: www.iwmi.org/assessment Institute ISSN 1391-9407 ISBN 92-9090-624 3 The Comprehensive Assessment of Water Management in Agriculture takes stock of the costs, benefits and impacts of the past 50 years of water development for agriculture, the water management challenges communities are facing today, and solutions people have developed. The results of the Assessment will enable farming communities, governments and donors to make better-quality investment and management decisions to meet food and environmental security objectives in the near future and over the next 25 years. The Research Report Series captures results of collaborative research conducted under the Assessment. It also includes reports contributed by individual scientists and organizations that significantly advance knowledge on key Assessment questions. Each report undergoes a rigorous peer-review process. The research presented in the series feeds into the Assessment’s primary output—a “State of the World” report and set of options backed by hundreds of leading water and development professionals and water users. Reports in this series may be copied freely and cited with due acknowledgement. Electronic copies of reports can be downloaded from the Assessment website (www.iwmi.org/assessment). If you are interested in submitting a report for inclusion in the series, please see the submission guidelines available on the Assessment website or send a written request to: Sepali Goonaratne, P.O. -

Diet Memoir Title New.Pmd
EDITORIAL BOARD Chief Patron : Sri Dadi Jagan Prabhakar Chairman Patron : Sri Dadi Ratnakar Secretary & Correspondent President : Dr. M. Venu Gopal Rao Principal Editor : Sri P.V. Murali Sr. Asst. Prof. Dept. of Humanities Co-Editors : Sri K. Sasidhar Asst. Prof. Dept. of Humanities Ms. S. Vanaja Asst. Prof. Dept. of Humanities MEMBERS Prof. Dr. K. Amarendra, Vice-Principal Ms. S.D.R.L. Pavani, Dept of Civil Mr. G. Jagadeesh, Dept of EEE Ms. M. Jyoti, Dept of ECE Mr K. Nookaraju, Dept of CSE Ms. SVGS Srujana, Dept of Humanities Ms. P. Athira, Dept of Management Mr. K. Ratnakar, Head, R&D am happy to learn that the Dadi Institute of Engineering and Technology has completed 10 glorious I years of its existence to celebrate with visible pride its Decennial, an important milestone in the life of an Institution dedicated to the cause of imparting quality technical education. Ever since its inception, this College is in the vanguard of upholding the high standards of professional dignity and decorum as per my knowledge goes. DIET as an institute is rising fast in stature, ranking, memberships to premier bodies and the success of its alumni in India and abroad points out the exceptional management and leadership skills of its faculty and students. My dear student friends I am happy in jotting a few lines for you through this college Magazine, Believe in quotes of Nelson Mandela "I learned that courage was not the absence of fear, but the triumph over it. The brave man is not he who does not feel afraid, but he who conquers that fear." And "Education is the most powerful weapon which you can use to change the world." Don't feel sad because you are different from others. -

Volume8 Issue2(1) 2019
Volume 8, Issue 2, February 2019 International Journal of Multidisciplinary Educational Research Published by Sucharitha Publications 48-12-3/7, Flat No: 302, Alekya Residency Srinagar, Visakhapatnam – 530 016 Andhra Pradesh – India Email: [email protected] Website: www.ijmer.in Editorial Board Editor-in-Chief Dr. K. Victor Babu Associate Professor, Institute of Education Mettu University, Metu, Ethiopia. EDITORIAL BOARD MEMBERS Prof. S.Mahendra Dev Prof. Igor Kondrashin Vice Chancellor The Member of The Russian Philosophical Indira Gandhi Institute of Development Society Research, Mumbai The Russian Humanist Society and Expert of The UNESCO, Moscow, Russia Prof.Y.C. Simhadri Vice Chancellor, Patna University Dr. Zoran Vujisiæ Former Director Rector Institute of Constitutional and Parliamentary St. Gregory Nazianzen Orthodox Institute Studies, New Delhi & Universidad Rural de Guatemala, GT, U.S.A Formerly Vice Chancellor of Benaras Hindu University, Andhra University Nagarjuna University, Patna University Prof.U.Shameem Department of Zoology Prof. (Dr.) Sohan Raj Tater Andhra University Visakhapatnam Former Vice Chancellor Singhania University, Rajasthan Dr. N.V.S.Suryanarayana Dept. of Education, A.U. Campus Prof.K.Sreerama Murty Vizianagaram Department of Economics Andhra University - Visakhapatnam Dr. Kameswara Sharma YVR Asst. Professor Dr.V.Venkateswarlu Dept. of Zoology Assistant Professor Sri. Venkateswara College, Delhi University, Dept. of Sociology & Social Work Delhi Acharya Nagarjuna University, Guntur I Ketut Donder Prof. P.D.Satya Paul Depasar State Institute of Hindu Dharma Department of Anthropology Indonesia Andhra University – Visakhapatnam Prof. Roger Wiemers Prof. Josef HÖCHTL Professor of Education Department of Political Economy Lipscomb University, Nashville, USA University of Vienna, Vienna & Ex. Member of the Austrian Parliament Dr. -
Andhra Pradesh State Administration Report
ANDHRA PRADESH STATE ADMINISTRATION REPORT 1982-83 / s it^-ogo^ki_ j CONTENTS Chapter N o .' Name o f the Chapter Pages (1) (2) (3) I The Chief Events o f the year 1—2 II The State and the Executive 3—8 III The Legislature • .. 9—11 IV Education Department 13—37 V Finance and Planning (Fin.Wing) Department 39 -^ 9 VI Finance and Planning (Plng.Wing) Department 51—55 V II General Administration Department 57—72 VIII Forest and Rural Development Department 73—91 IX ^Food and Agriculture Department .. 93—131 X Industries and Commerce Department 133— 145 XI • Housing, Municipal Administration and Urban Development Department .. 147—165 XII Home Department 167—193 XIII Irrigation Department 195—215 XIV Labour, Employment, Nutrition and Technical Education Department 217—243 XV Irrigation, Utilisation and Command Area Deve lopment Department 245—255 XVI Medical and Health’ Department 257—280 x v n Panchayati Raj Department • .. 281—284 XVIII Revenue Department 285—297 XIX Social Welfare Department 299—321 XX Transport, Roads and Buildings Department 323—333 iii C hapter-I CHIEF EVENTS OF THE YEAR 1982-83 A p ril 1982 11 Dr. M. Chenna Reddy appointed as Governor of Punjab. 21 Government’s decision to preserve Kandukuri’s house in Ryahmundry as n V o n il monument announced. M ay 16 First Kidney ransplantation performed in the Osmania General Hospital. 19 CAPOL, Chirala, a Joint venture of the APID C bags export award for 1981-82. 20 First mobile hospi al inaugurated in "he Ranga Reddy Distric’ . 25 Water and Land Management Training and Re search Institute, with World Bank aid, se' up in Hyderabad. -

Anil Neerukonda Institute of Technology & Sciences
ANIL NEERUKONDA INSTITUTE OF TECHNOLOGY & SCIENCES (Affiliated to AU, Approved by AICTE & Accredited by NBA) SANGIVALASA-531 162, Bheemunipatnam Mandal, Visakhapatnam District Phone: 08933-225083/84/87 Fax: 226395 MANDATORY DISCLOSURE Mandatory Disclosure updated on : 14-12-2011 1. AICTE File No. : 1-3992214 Date of First Approval : F.No:730-50-32(E)/ET/2001 2. Name of the Institution : Anil Neerukonda Institute of Technology & Sciences Address of the Institution : Sangivalasa Bheemunipatnam Mandal City & Pin Code : VISAKHAPATNAM – 531 162 State / UT : Andhra Pradesh Longitude & Latitude : Phone number with STD Code : 08933-225083, 84 & 85 Fax number with STD Code : 08933-226395 Office hours at the Institution : 08:30 AM to 05:30 PM Academic hours in the Institution : 09:30 AM to 04:10 PM Email : [email protected] Website : www.anits.edu.in Nearest Railway Station (Dist in KM) : Visakhapatnam (27 KM) Nearest Airport (Dist in KM) : Visakhapatnam (40 KM) 3. Type of Institution : Private – Self Financed Category (1) of the Institution : Non Minority Category (2) of the Institution : Co-Ed 4. Name of the Organization running the Institution : Anil Neerukonda Educational Society Type of the Organization : Society Address of the Association : 10-50-18/1(15), 2nd Floor, Siripuram Towers, Siripuram Junction, Visakhapatnam, Andhra Pradesh, India Registered with : Societies Registration Act 1860 (Act. XXI of 1860) Registration Date : 02-08-2000 Website of the Organization : www.anits.edu.in 5. Name of the affiliating University : Andhra University Address : Visakhapatnam Website : www.andhrauniversity.info Latest affiliation period : Permanent Affiliation granted in 2009 6. Name of the Principal : Prof.