ER-LA FDA REMS Modification Review, April 12, 2015
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Oxycontin®) and Oxymorphone (Opana® ER) Reference Number: ERX.NSST.17 Effective Date: 06/15 Revision Log Last Review Date: 09/16
Clinical Policy: extended-release oxycodone (OxyContin®) and oxymorphone (Opana® ER) Reference Number: ERX.NSST.17 Effective Date: 06/15 Revision Log Last Review Date: 09/16 Clinical policies are intended to be reflective of current scientific research and clinical thinking. This policy is current at the time of approval, may be updated and therefore is subject to change. This Clinical Policy is not intended to dictate to providers how to practice medicine, nor does it constitute a contract or guarantee regarding payment or results. Providers are expected to exercise professional medical judgment in providing the most appropriate care, and are solely responsible for the medical advice and treatment of members. This policy is the property of Envolve Pharmacy Solutions. Unauthorized copying, use, and distribution of this Policy or any information contained herein is strictly prohibited. By accessing this policy, you agree to be bound by the foregoing terms and conditions, in addition to the Site Use Agreement for Health Plans associated with Envolve Pharmacy Solutions. Description The intent of the criteria is to ensure that patients follow selection elements established by Envolve Pharmacy Solutions for the use of extended-release oxycodone (OxyContin®) and oxymorphone (Opana® ER). Policy/Criteria It is the policy of health plans affiliated with Envolve Pharmacy Solutions® that extended-release oxycodone (OxyContin®) and oxymorphone (Opana® ER) are medically necessary for members meeting the following criteria: Initial Approval Criteria (must meet all): A. Failure of an immediate-release (short-acting) narcotic analgesic; B. Failure of fentanyl patch and morphine extended-release tablets/capsules in the past 6 months, unless intolerant or contraindicated; C. -

Veterinary Anesthetic and Analgesic Formulary 3Rd Edition, Version G
Veterinary Anesthetic and Analgesic Formulary 3rd Edition, Version G I. Introduction and Use of the UC‐Denver Veterinary Formulary II. Anesthetic and Analgesic Considerations III. Species Specific Veterinary Formulary 1. Mouse 2. Rat 3. Neonatal Rodent 4. Guinea Pig 5. Chinchilla 6. Gerbil 7. Rabbit 8. Dog 9. Pig 10. Sheep 11. Non‐Pharmaceutical Grade Anesthetics IV. References I. Introduction and Use of the UC‐Denver Formulary Basic Definitions: Anesthesia: central nervous system depression that provides amnesia, unconsciousness and immobility in response to a painful stimulation. Drugs that produce anesthesia may or may not provide analgesia (1, 2). Analgesia: The absence of pain in response to stimulation that would normally be painful. An analgesic drug can provide analgesia by acting at the level of the central nervous system or at the site of inflammation to diminish or block pain signals (1, 2). Sedation: A state of mental calmness, decreased response to environmental stimuli, and muscle relaxation. This state is characterized by suppression of spontaneous movement with maintenance of spinal reflexes (1). Animal anesthesia and analgesia are crucial components of an animal use protocol. This document is provided to aid in the design of an anesthetic and analgesic plan to prevent animal pain whenever possible. However, this document should not be perceived to replace consultation with the university’s veterinary staff. As required by law, the veterinary staff should be consulted to assist in the planning of procedures where anesthetics and analgesics will be used to avoid or minimize discomfort, distress and pain in animals (3, 4). Prior to administration, all use of anesthetics and analgesic are to be approved by the Institutional Animal Care and Use Committee (IACUC). -

Heterodimerization of Μ and Δ Opioid Receptors: a Role in Opiate Synergy
The Journal of Neuroscience, 2000, Vol. 20 RC110 1of5 Heterodimerization of and ␦ Opioid Receptors: A Role in Opiate Synergy I. Gomes, B. A. Jordan, A. Gupta, N. Trapaidze, V. Nagy, and L. A. Devi Departments of Pharmacology and Anesthesiology, New York University School of Medicine, New York, New York 10016 Opiate analgesics are widely used in the treatment of severe -selective ligands results in a significant increase in the bind- pain. Because of their importance in therapy, different strate- ing of a ␦ receptor agonist. This robust increase is also seen in gies have been considered for making opiates more effective SKNSH cells that endogenously express both and ␦ recep- while curbing their liability to be abused. Although most opiates tors. Furthermore, we find that a ␦ receptor antagonist en- exert their analgesic effects primarily via opioid receptors, a hances both the potency and efficacy of the receptor signal- number of studies have shown that ␦ receptor-selective drugs ing; likewise a antagonist enhances the potency and efficacy can enhance their potency. The molecular basis for these find- of the ␦ receptor signaling. A combination of agonists ( and ␦ ings has not been elucidated previously. In the present study, receptor selective) also synergistically binds and potentiates we examined whether heterodimerization of and ␦ receptors signaling by activating the –␦ heterodimer. Taken together, could account for the cross-modulation previously observed these studies show that heterodimers exhibit distinct ligand between these two receptors. We find that co-expression of binding and signaling characteristics. These findings have im- and ␦ receptors in heterologous cells followed by selective portant clinical ramifications and may provide new foundations immunoprecipitation results in the isolation of –␦ het- for more effective therapies. -

Modulation of Opioid Transport at the Blood-Brain Barrier by Altered ATP-Binding Cassette (ABC) Transporter Expression and Activity
pharmaceutics Review Modulation of Opioid Transport at the Blood-Brain Barrier by Altered ATP-Binding Cassette (ABC) Transporter Expression and Activity Junzhi Yang 1, Bianca G. Reilly 2, Thomas P. Davis 1,2 and Patrick T. Ronaldson 1,2,* 1 Department of Pharmacology and Toxicology, College of Pharmacy, University of Arizona, 1295 N. Martin St., P.O. Box 210207, Tucson, AZ 85721, USA; [email protected] (J.Y.); [email protected] (T.P.D.) 2 Department of Pharmacology, College of Medicine, University of Arizona, 1501 N. Campbell Ave, P.O. Box 245050, Tucson, AZ 85724-5050, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-520-626-2173 Received: 19 September 2018; Accepted: 16 October 2018; Published: 18 October 2018 Abstract: Opioids are highly effective analgesics that have a serious potential for adverse drug reactions and for development of addiction and tolerance. Since the use of opioids has escalated in recent years, it is increasingly important to understand biological mechanisms that can increase the probability of opioid-associated adverse events occurring in patient populations. This is emphasized by the current opioid epidemic in the United States where opioid analgesics are frequently abused and misused. It has been established that the effectiveness of opioids is maximized when these drugs readily access opioid receptors in the central nervous system (CNS). Indeed, opioid delivery to the brain is significantly influenced by the blood-brain barrier (BBB). In particular, ATP-binding cassette (ABC) transporters that are endogenously expressed at the BBB are critical determinants of CNS opioid penetration. In this review, we will discuss current knowledge on the transport of opioid analgesic drugs by ABC transporters at the BBB. -
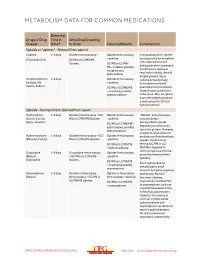
Metabolism Data for Common Medications
METABOLISM DATA FOR COMMON MEDICATIONS Detection Drugs or Drug Time in Urine Drug Screening Classes Urine* to Order Expected Results Consideration Opioids or “opiates” – Natural (from opium) Codeine 1–3 days Opiates Immunoassay + Opiates Immunoassay Immunoassays for “opiates” are responsive for morphine (Tylenol #2/3/4) GC/MS or LC/MS/MS – positive and codeine but do not Opiates GC/MS or LC/MS/ distinguish which is present. MS – codeine, possibly Confirmatory testing is morphine and required to reliably identify hydrocodone drug(s) present. Since Morphine (Avinza, 1–3 days Opiates Immunoassay codeine is metabolized Embeda, MS – positive to morphine and small Contin, Kadian) GC/MS or LC/MS/MS quantities to hydrocodone, – morphine, possibly these drugs may be found hydromorphone in the urine. Also, morphine may metabolize to produce a small amount (<10%) of hydromorphone. Opioids – Semisynthetic (derived from opium Hydrocodone 1–3 days Opiates Immunoassay + GC/ Opiates Immunoassay “Opiates” immunoassays (Lorcet, Lortab, MS or LC/MS/MS Opiates – positive may also detect Norco, Vicodin) semisynthetic opioids GC/MS or LC/MS/MS – depending on their cross- hydrocodone, possibly reactivity pattern. However, hydromorphone a negative result does not Hydromorphone 1–3 days Opiates Immunoassay + GC/ Opiates Immunoassay exclude use of semisynthetic (Dilaudid, Exalgo) MS or LC/MS/MS Opiates –positive opioids. Confirmatory GC/MS or LC/MS/MS testing (GC/MS or LC/ – hydromorphone MS/MS) is required to verify compliance with the Oxycodone 1–3 days Oxycodone Immunoassay Opiates Immunoassay prescribed semisynthetic (Roxicet, + GC/MS or LC/MS/MS –positive opioid(s). OxyContin) Opiates GC/MS or LC/MS/MS Since hydrocodone is – oxycodone possibly metabolized in small oxymorphone amounts to hydromorphone, Oxymorphone 1–3 days Opiates or Oxycodone Opiates or Oxycodone both may be found in (Opana) Immunoassay + GC/MS or Immunoassay – positive the urine. -

Summary of Synthetic Opioid Testing Within the Department of Defense
SUMMARY OF SYNTHETIC OPIOID TESTING WITHIN THE DEPARTMENT OF DEFENSE Tom Martin, Ph.D. Liaison to Director, DoD Drug Testing and Program Policy PERSONNEL AND READINESS PERSONNEL AND READINESS Drug Demand Reduction Program Mission and Scope Mission: Enable operational readiness, safety, and security of the Total Force by deterring illicit and prescription drug abuse through robust and dynamic drug testing; emerging drug threat surveillance; prevention, education, and outreach efforts; and development of new testing procedures. Scope: All DoD components and DoD civilians in testing designated positions (TDPs) Policies: DODI 1010.01 “Military Personnel Drug Abuse Testing Program (MPDATP)” DODI 1010.09 “DoD Civilian Employee Drug-Free Workplace Program” DODI 1010.16 “Technical Procedures for the Military Personnel Drug Abuse Testing Program (MPDATP)” PERSONNEL AND READINESS DDRP Driving Factors • Drug abuse in the general U.S. 18-25 year old male group is estimated to be 17-20%– the population from which the Service recruits their enlisted personnel • Before DoD instituted drug testing among Service personnel, drug use was a significant recurring problem – Vietnam (estimated over 5% of returning service members addicted to heroin) – 1981 CVN Nimitz aviation mishap – 14 killed, 48 injured, 7 aircraft destroyed, 11 aircraft damaged, $150M in damages, six deceased with detectable levels of marijuana • Notable increase in abuse/misuse of prescription pain medications • Personnel abusing illicit drugs or prescription medications are a safety hazard -

Hydromorphone Hydrochloride) C-II
NDA 19-034/S-018-Label Page 1 DILAUDID® and DILAUDID-HP® INJECTION 1 mg/mL, 2 mg/mL, 4mg/mL, and 10 mg/mL (hydromorphone hydrochloride) C-II WARNING: DILAUDID-HP (high potency, 10 mg/mL ampules and vials) is a more concentrated solution of hydromorphone than DILAUDID INJECTION, and is intended for use only in opioid-tolerant patients. Do not confuse DILAUDID-HP with standard parenteral formulations of DILAUDID or other opioids, as overdose and death could result. DILAUDID® INJECTION (1, 2, and 4 mg/mL ampules, sterile solution for parenteral administration) and DILAUDID-HP® contain hydromorphone, a potent Schedule II opioid agonist. Schedule II opioid agonists, including morphine, oxymorphone, hydromorphone, oxycodone, fentanyl and methadone, have the highest potential for abuse and risk of producing respiratory depression. Ethanol, other opioids, and other central nervous system depressants (e.g., sedative-hypnotics, skeletal muscle relaxants) can potentiate the respiratory- depressant effects of hydromorphone and increase the risk of adverse outcome, including death. DESCRIPTION DILAUDID (hydromorphone hydrochloride), a hydrogenated ketone of morphine, is an opioid analgesic. The chemical name of DILAUDID (hydromorphone hydrochloride) is 4,5α-epoxy-3-hydroxy-17-methylmorphinan-6-one hydrochloride. The structural formula is: NDA 19-034/S-018-Label Page 2 M.W. 321.8 DILAUDID INJECTION is available in ampules for parenteral administration. Each 1 mL of sterile solution contains 1 mg, 2 mg, or 4 mg hydromorphone hydrochloride with 0.2% sodium citrate and 0.2% citric acid solution. DILAUDID INJECTION ampules are sterile. HIGH POTENCY DILAUDID (DILAUDID-HP) is available in AMBER ampules or single dose vials for intravenous (IV), subcutaneous (SC), or intramuscular (IM) administration. -

Summary Report of Benefit-Risk Assessment NALDEBAIN
Summary Report of Benefit-Risk Assessment NALDEBAIN EXTENDED RELEASE INJECTION 75 MG/ML NEW DRUG APPLICATION Active Ingredient(s) Dinalbuphine sebacate Product Registrant Intega Pte Ltd Product Registration Number SIN16058P Application Route Abridged evaluation Date of Approval 15 December 2020 Copyright © 2021 Health Sciences Authority of Singapore You may download, view, print and reproduce this summary report without modifications for non-commercial purposes only. Except as otherwise provided, the contents of this summary report may not be reproduced, republished, uploaded, posted, transmitted or otherwise distributed in any way without the prior written permission of the Health Sciences Authority. This summary report and its contents are made available on an “as is” basis and the Health Sciences Authority makes no warranty of any kind, whether express or implied. The information in the summary report is provided for general information only and the contents of the summary report do not constitute medical or other professional advice. If medical or other professional advice is required, services of a competent professional should be sought. Table of Contents A INTRODUCTION ............................................................................................................. 3 B ASSESSMENT OF PRODUCT QUALITY ....................................................................... 3 C ASSESSMENT OF CLINICAL EFFICACY ...................................................................... 4 D ASSESSMENT OF CLINICAL SAFETY ......................................................................... -

Use of Chronic Opioid Therapy in Chronic Noncancer Pain CHRONIC NONCANCER PAINNONCANCER CHRONIC Evidence Review
CLINICAL GUIDELINE FOR THE USE OF CHRONIC OPIOID THERAPY IN CLINICAL GUIDELINE FOR THE USE OF CHRONIC OPIOID THERAPY IN GUIDELINE FOR THE Use of Chronic Opioid Therapy in Chronic Noncancer Pain CHRONIC NONCANCER PAIN Evidence Review The American Pain Society in Conjunction with The American Academy of Pain Medicine EVIDENCE REVIEW APS-AAPM Clinical Guidelines for the Use of Chronic Opioid Therapy in Chronic Noncancer Pain TABLE OF CONTENTS Page Introduction 1 Purpose of evidence review ...................................................................... 1 Background 1 Previous guidelines ................................................................................... 2 Scope of evidence review 3 Key questions............................................................................................ 3 Populations................................................................................................ 7 Interventions.............................................................................................. 8 Outcomes.................................................................................................. 8 Conflict of interest............................................................................................. 10 Methods 10 Literature search and strategy................................................................... 10 Inclusion and exclusion criteria.................................................................. 11 Data extraction and synthesis .................................................................. -

Lessons Learned from Precipitated Withdrawal After Early Buprenorphine Initiation After Methadone Elaine Rigney MD, Timothy Wiegand MD
‘Start Low & Delay Until in Withdrawal’ –Lessons Learned from Precipitated Withdrawal after Early Buprenorphine Initiation after Methadone Elaine Rigney MD, Timothy Wiegand MD Background: Buprenorphine is a potent partial agonist at the mu-opioid receptor. It is generally considered a safe drug , and has become increasingly available for pain, typically in lower-dose formulations, or off-label prescribing using standard formulations for opioid dependence. Despite its safety profile, toxicity can occur in patients taking long-acting or sustained-release opioids. We describe a case of severe precipitated withdrawal induced by high doses of buprenorphine without adequate wash-out from methadone and SR oxymorphone. We also discuss treatment for patients experiencing precipitated opioid withdrawal. Case report A 55 year-old male with PMH of prostate cancer with debilitating chronic post-surgical pain and radiation-induced neuropathy received gradually escalating doses of methadone and oxymorphone without significant relief. He was taking 70mg/day methadone and 200mg/day oxymorphone. His Xwaivered pain provider proposed transition to buprenorphine. The patient brought prescribed buprenorphine/naloxone doses to the pain clinic for induction after instructions to stop his methadone 18 hours prior (30 mg noon dose and 20 mg in the AM) and to take the last SR oxymorphone 40 mg dose the morning prior to induction. The patient was told to take 8 mg buprenorphine and, “15 minutes later ‘my’ ears started to get warm and he told me to take another 4 mg.” Shortly after he developed severe diffuse pain, cramping, muscle spasms and myoclonic jerks. His wife reported, “he was flailing his arms out and screaming for help!” The clinic physician administered midazolam and another buprenorphine 4mg but his symptoms worsened and EMS was called. -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

Health Alert Movement 2019: Detroit Electronic Music Festival Hart Plaza Downtown Detroit May 25-28, 2019
Health Alert Movement 2019: Detroit Electronic Music Festival Hart Plaza Downtown Detroit May 25-28, 2019 In this issue Updates for 2019 .............................................................................................................. 2 Prehospital Concerns ....................................................................................................... 3 Hospital Concerns ............................................................................................................ 4 THC .................................................................................................................................. 5 THC Homologs ................................................................................................................. 5 Stimulants......................................................................................................................... 7 Opioids ........................................................................................................................... 10 GABA-B .......................................................................................................................... 12 NMDA ............................................................................................................................. 13 Inhalants ......................................................................................................................... 14 Other Hallucinogens ......................................................................................................