COS: Chemistry in the Earth System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
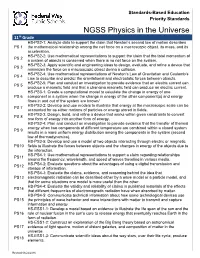
NGSS Physics in the Universe
Standards-Based Education Priority Standards NGSS Physics in the Universe 11th Grade HS-PS2-1: Analyze data to support the claim that Newton’s second law of motion describes PS 1 the mathematical relationship among the net force on a macroscopic object, its mass, and its acceleration. HS-PS2-2: Use mathematical representations to support the claim that the total momentum of PS 2 a system of objects is conserved when there is no net force on the system. HS-PS2-3: Apply scientific and engineering ideas to design, evaluate, and refine a device that PS 3 minimizes the force on a macroscopic object during a collision. HS-PS2-4: Use mathematical representations of Newton’s Law of Gravitation and Coulomb’s PS 4 Law to describe and predict the gravitational and electrostatic forces between objects. HS-PS2-5: Plan and conduct an investigation to provide evidence that an electric current can PS 5 produce a magnetic field and that a changing magnetic field can produce an electric current. HS-PS3-1: Create a computational model to calculate the change in energy of one PS 6 component in a system when the change in energy of the other component(s) and energy flows in and out of the system are known/ HS-PS3-2: Develop and use models to illustrate that energy at the macroscopic scale can be PS 7 accounted for as either motions of particles or energy stored in fields. HS-PS3-3: Design, build, and refine a device that works within given constraints to convert PS 8 one form of energy into another form of energy. -

Intercommutation of U(1) Global Cosmic Strings
Prepared for submission to JHEP Intercommutation of U(1) global cosmic strings Guy D. Moore Institut f¨urKernphysik, Technische Universit¨atDarmstadt Schlossgartenstraße 2, D-64289 Darmstadt, Germany E-mail: [email protected] Abstract: Global strings (those which couple to Goldstone modes) may play a role in cosmology. In particular, if the QCD axion exists, axionic strings may control the efficiency of axionic dark matter abundance. The string network dynamics depend on the string intercommutation efficiency (whether strings re-connect when they cross). We point out that the velocity and angle in a collision between global strings \renormalize" between the network scale and the microscopic scale, and that this plays a significant role in their intercommutation dynamics. We also point out a subtlety in treating intercommutation of very nearly antiparallel strings numerically. We find that the global strings of a O(2)- breaking scalar theory do intercommute for all physically relevant angles and velocities. Keywords: axions, dark matter, cosmic strings, global strings arXiv:1604.02356v1 [hep-ph] 8 Apr 2016 Contents 1 Introduction1 2 Global string review3 3 Renormalization of string angle and velocity5 4 Microscopic study of intercommutation9 5 Discussion and Conclusions 12 1 Introduction fsec:introg Cosmic strings [1,2] are hypothetical extended solitonic excitations which may play a sig- nificant role in cosmology. Their original motivation, for structure formation [3{5], appears in conflict with modern microwave sky data [6]. But cosmic strings may be important in other contexts. In particular, if the QCD axion [7,8] exists, the axion field may contain a string network in the early Universe [9] which may dominate axion production and play a central role in the axion as a dark matter candidate. -

Chemistry in the Earth System HS Models: Handout 3
High School 3 Course Model: Chemistry in the Earth System HS Models: Handout 3 Guiding Questions Chemistry in the Earth System Performance Expectations Instructional What is energy, how is it HS-PS1-3. Plan and conduct an investigation to gather evidence to compare the structure of substances at the bulk scale to infer the Segment 1: measured, and how does it strength of electrical forces between particles. [Clarification Statement: Emphasis is on understanding the strengths of forces between Combustion flow within a system? particles, not on naming specific intermolecular forces (such as dipole-dipole). Examples of particles could include ions, atoms, molecules, What mechanisms allow us and networked materials (such as graphite). Examples of bulk properties of substances could include the melting point and boiling point, to utilize the energy of our vapor pressure, and surface tension.] [Assessment Boundary: Assessment does not include Raoult’s law calculations of vapor pressure.] foods and fuels? (Introduced, but not assessed until IS3) HS-PS1-4. Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy. [Clarification Statement: Emphasis is on the idea that a chemical reaction is a system that affects the energy change. Examples of models could include molecular-level drawings and diagrams of reactions, graphs showing the relative energies of reactants and products, and representations showing energy is conserved.] [Assessment Boundary: Assessment does not include calculating the total bond energy changes during a chemical reaction from the bond energies of reactants and products.] (Introduced, but not assessed until IS4) HS-PS1-7. -

Reduced Order Modelling of Streamers and Their Characterization by Macroscopic Parameters by Colin A
Reduced order modelling of streamers and their characterization by macroscopic parameters by Colin A. Pavan BASc, University of Waterloo (2017) Submitted to the Department of Aeronautical and Astronautical Engineering in partial fulfillment of the requirements for the degree of Master of Science in Aeronautical and Astronautical Engineering at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY June 2019 ○c Massachusetts Institute of Technology 2019. All rights reserved. Author................................................................ Department of Aeronautical and Astronautical Engineering May 21, 2019 Certified by. Carmen Guerra-Garcia Assistant Professor of Aeronautics and Astronautics Thesis Supervisor Accepted by........................................................... Sertac Karaman Associate Professor of Aeronautics and Astronautics Chair, Graduate Program Committee 2 Reduced order modelling of streamers and their characterization by macroscopic parameters by Colin A. Pavan Submitted to the Department of Aeronautical and Astronautical Engineering on May 21, 2019, in partial fulfillment of the requirements for the degree of Master of Science in Aeronautical and Astronautical Engineering Abstract Electric discharges in gases occur at various scales, and are of both academic and prac- tical interest for several reasons including understanding natural phenomena such as lightning, and for use in industrial applications. Streamers, self-propagating ioniza- tion fronts, are a particularly challenging regime to study. They are difficult to study computationally due to the necessity of resolving disparate length and time scales, and existing methods for understanding single streamers are impractical for scaling up to model the hundreds to thousands of streamers present in a streamer corona. Conversely, methods for simulating the full streamer corona rely on simplified models of single streamers which abstract away much of the relevant physics. -

A Two-Scale Approach to Logarithmic Sobolev Inequalities and the Hydrodynamic Limit
Annales de l’Institut Henri Poincaré - Probabilités et Statistiques 2009, Vol. 45, No. 2, 302–351 DOI: 10.1214/07-AIHP200 © Association des Publications de l’Institut Henri Poincaré, 2009 www.imstat.org/aihp A two-scale approach to logarithmic Sobolev inequalities and the hydrodynamic limit Natalie Grunewalda,1, Felix Ottob,2, Cédric Villanic,3 and Maria G. Westdickenbergd,4 aInstitute für Angewandte Mathematik, Universität Bonn. E-mail: [email protected] bInstitute für Angewandte Mathematik, Universität Bonn. E-mail: [email protected] cUMPA, École Normale Supérieure de Lyon, and Institut Universitaire de France. E-mail: [email protected] dSchool of Mathematics, Georgia Institute of Technology. E-mail: [email protected] Received 30 May 2007; accepted 7 November 2007 Abstract. We consider the coarse-graining of a lattice system with continuous spin variable. In the first part, two abstract results are established: sufficient conditions for a logarithmic Sobolev inequality with constants independent of the dimension (Theorem 3) and sufficient conditions for convergence to the hydrodynamic limit (Theorem 8). In the second part, we use the abstract results to treat a specific example, namely the Kawasaki dynamics with Ginzburg–Landau-type potential. Résumé. Nous étudions un système sur réseau à variable de spin continue. Dans la première partie, nous établissons deux résultats abstraits : des conditions suffisantes pour une inégalité de Sobolev logarithmique avec constante indépendante de la dimension (Théorème 3), et des conditions suffisantes pour la convergence vers la limite hydrodynamique (Theorème 8). Dans la seconde partie, nous utilisons ces résultats abstraits pour traiter un exemple spécifique, à savoir la dynamique de Kawasaki avec un potentiel de type Ginzburg–Landau. -

Time Scales for Rounding of Rocks Through Stochastic Chipping
Time Scales for Rounding of Rocks through Stochastic Chipping D. J. Priour, Jr1 1Department of Physics & Astronomy, Youngstown State University, Youngstown, OH 44555, USA (Dated: March 10, 2020) For three dimensional geometries, we consider stones (modeled as convex polyhedra) subject to weathering with planar slices of random orientation and depth successively removing material, ultimately yielding smooth and round (i.e. spherical) shapes. An exponentially decaying acceptance probability in the area exposed by a prospective slice provides a stochastically driven physical basis for the removal of material in fracture events. With a variety of quantitative measures, in steady state we find a power law decay of deviations in a toughness parameter γ from a perfect spherical shape. We examine the time evolution of shapes for stones initially in the form of cubes as well as irregular fragments created by cleaving a regular solid many times along random fracture planes. In the case of the former, we find two sets of second order structural phase transitions with the usual hallmarks of critical behavior. The first involves the simultaneous loss of facets inherited from the parent solid, while the second transition involves a shift to a spherical profile. Nevertheless, for mono-dispersed cohorts of irregular solids, the loss of primordial facets is not simultaneous but occurs in stages. In the case of initially irregular stones, disorder obscures individual structural transitions, and relevant observables are smooth with respect to time. More broadly, we find that times for the achievement of salient structural milestones scale quadratically in γ. We use the universal dependence of variables on the fraction of the original volume remaining to calculate time dependent variables for a variety of erosion scenarios with results from a single weathering scheme such as the case in which the fracture acceptance probability depends on the relative area of the prospective new face. -

Chapter1describing the Physical Universe
Chapter 1 Describing the Physical Universe On June 21, 2004, SpaceShipOne became the first private aircraft to leave Earth's atmosphere and enter space. What is the future of this technology? Private companies are hoping to sell tickets to adventurous people who want to take a trip beyond Earth's atmosphere. Our study of physics can help us understand how such a trip is possible. Many people, when asked the question “What is physics?” respond with “Oh, physics is all about complicated math equations and confusing laws to memorize.” This response may describe the physics studied in some classrooms, but this is not the physics of the world around us, and this is definitely not the physics that you will study in this Key Questions course! In this chapter you will be introduced to what 3 Does air have mass and take up studying physics is REALLY all about, and you will space? What about light? begin your physics journey by studying motion and 3 How can an accident or mistake speed. lead to a scientific discovery? 3 What is the fastest speed in the universe? 3 1.1 What Is Physics? Vocabulary What is physics and why study it? Many students believe physics is a complicated set of rules, natural law, experiment, analysis, equations to memorize, and confusing laws. Although this is sometimes the way physics is taught, mass, system, variable, it is not a fair description of the science. In fact, physics is about finding the simplest and least macroscopic, scientific method, complicated explanation for things. It is about observing how things work and finding the independent variable, dependent connections between cause and effect that explain why things happen. -

Length, Mass, and Time
5/13/14 Objectives Length, mass, • Record data using scientific notation. and time • Record data using International System (SI) units. Assessment Assessment 1. Express the following numbers in scientific notation: 2. Which of the following data are recorded using International System (SI) units? a. 275 a. 107 meters b. 0.00173 b. 24.5 inches c. 93,422 c. 5.8 × 102 pounds d. 0.000018 d. 26.3 kilograms e. 17.9 seconds Physics terms Physics terms • measurement • scale • matter • macroscopic • mass • microscopic • length • temperature • surface area • scientific notation • volume • exponent • density 1 5/13/14 Equations The International System of units Physicists commonly use the International System (SI) to measure and describe the density: world. This system consists of seven fundamental quantities and their metric units. The International System of units Mass, length, and time Physicists commonly use the International Mass describes the quantity of matter. System (SI) to measure and describe the world. Language: “The store had a massive blow-out sale this weekend!” This system consists of seven fundamental quantities and their metric How is the term “massive” incorrectly used units. in the physics sense? Why is it incorrect? Can you suggest more correct words? The three fundamental quantities needed for the study of mechanics are: mass, length, and time. Mass, length, and time Mass, length, and time Length describes the quantity of space, Time describes the flow of the universe from such as width, height, or distance. the past through the present into the future. In physics this will usually mean a quantity Language: “How long are you going to be in of time in seconds, such as 35 s. -

A General Way of Obtaining Macroscopic Description from Microscopic Variables
From Micro to Macromechanics: A General Way of Obtaining Macroscopic Description from Microscopic Variables M. Holecek Faculty of Applied Sciences University of West Bohemia, CZ-30614 Pilsen, Czech Republic [email protected]. cz I. SUMMARY A deformation of a macroscopic body is described at a microscopic level. Using the concept of an external, macroscopic control of the system a set of special "modes" of micro-deformations being sensitive on macroscopic influence is found out. These "modes" give a set of macroscopic variables enlarging the standard description of deformable media. The problem of microscopic relaxation is described by these new variables as an illustration. 2. INTRODUCTION Any variable used at macroscopic scales is an averaged value of some microscopic (mesoscopic) variables. These 'micro-variables', however, are usually not explicitly introduced and the description is formulated directly at a macroscopic scale without looking for a bridge connecting macroscopic variables with the microscopic ones. Nevertheless, the very question which macroscopic variables should be used can be deeply connected with the formulation of the problem at a microscopic level. In this study we propose a general approach of obtaining macroscopic variables from the field describing the micro-deformation of the body. The method is based on the fact that "macroscopic" is defined by an external influence on the body - if the body is under some macroscopic external conditions we are interested about a macroscopic reaction. This reaction, however, can depend on microscopic variables. The proposed method enables us to find some microscopic degrees of freedom or "modes" of micro-deformations that are sensitive to a defined macroscopic influence. -

2005Ikegami-Obs-Enha
Observation of enormously enhanced nuclear fusion in metallic Li liquid H lkegami R Pettersson L Einarsson Rapporterna kan bestallas frin Studsvikbiblioteket, 6 11 82 Nykoping. Tel0155-22 10 84. Fax 0155-26 30 44 Statens energimyndighet e-post [email protected] Box 3 10, 63 1 04 Eskilstuna Observation of enormously enhanced nuclear fusion in metallic Li liquid Fiirfattare: Hidetsugu Ikegami Hibariga-oka 2- 12-50, Takarazuka, Japan Dep of Analytical Chemistry, Uppsala University Roland Pettersson Dep of Analytical Chemistry, Uppsala University Lars Einarsson The Svedberg Laboratory, Uppsala University RAPPORT INOM OM~DETENERGIFORSKNING ALLMANT Rapportnummer: EFA 0512 Projektledare: Roland Pettersson Projektnummer : P2062 8 - 1 Projekthandlaggare pi Statens Energimyndighet: Lars TegnCr Box 310 -631 04 Esk~lstuna. Besoksadress Kungsgatan 43 Telefon 016-54420 00 . Telefax 016-54420 99 stem@stem se .www stemse Org nr 202100-5000 Observation of enormously enhanced nuclear fusion in metallic Li liquid Hidetsugu Ikegami * +, Roland Pettersson + & Lars ~inarsson* * Hibariga-oka 2-1 2-50, Takarazuka 665-0805, Japan + Department of Analytical Chemistry, Uppsala University, Box 599, SE-751 24 Uppsala, Sweden * The Svedberg Laboratory, Uppsala University, Box 533, SE-75121, Uppsala, Sweden Deuterons of some tens keV energy have been implanted on a surface of metallic Li liquid. Alpha particles produced in the fusion reaction 'Li + d + 8~e+ n + 201 + n were identified using Si surface barrier detectors (SSD) and thin foil energy loss method. The rate of alpha particles was up to one million per second at 1 pA of deuterons. This is a factor of 1010 - 1015 higher than what is expected based on available nuclear-reaction cross sections l. -

Biofilm Growth in Porous Media: Derivation of a Macroscopic Model from the Physics … 171
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Repositorio da Universidade da Coruña BIOFILM GROWTH IN POROUS MEDIA: DERIVATION OF A MACROSCOPIC MODEL FROM THE PHYSICS … 171 Biofilm growth in porous media: derivation of a macroscopic model from the physics at the pore scale via homogenization A. Philippe1,2, C. Geindreau1, P. Séchet2 and J. Martins3 1 Lab. 3S, CNRS-UJF-INPG, BP 53X, 38041 Grenoble cedex 9, France 2 LEGI, CNRS-UJF-INPG, BP 53X, 38041 Grenoble cedex 9, France 3 LTHE, CNRS-UJF-INPG, BP 53X, 38041 Grenoble cedex 9, France ABSTRACT. This paper concerns the modelling of biofilm in porous media. A macroscopic model (at the Darcy’s scale) is derived from the physics at the pore scale by using an upscaling technique, namely the homogenisation method of multiple scale expansions. The domain of validity of this macroscopic description depends on the order of magnitude of different dimensionless numbers arising from the description at the microscopic scale. We show that at the macroscopic scale (i) the flow is described by the classical Darcy's law (ii) the mass balance of the fluid includes a source term due to the biomass growth (iii) the transport of substrate is describe by a diffusion-advection-reaction equation (iv) the macroscopic biomass balance is given by a diffusion-reaction relation. The macroscopic reactive term is similar to the microscopic one. The homogenization process also shows that all the effective coefficients (permeability, effective diffusion tensors) arising in the macroscopic description depends on the biomass fraction. -
Physical Chemistry: Macroscopic Scale: Microscopic Scale (For CBSE, ICSE, IAS, NET, NRA 2022)
9/22/2021 Title: Physical Chemistry- FlexiPrep FlexiPrep Physical Chemistry: Macroscopic Scale: Microscopic Scale (For CBSE, ICSE, IAS, NET, NRA 2022) Get top class preparation for competitive exams right from your home: get questions, notes, tests, video lectures and more- for all subjects of your exam. Title: Physical Chemistry Physical Chemistry 1 of 6 9/22/2021 Title: Physical Chemistry- FlexiPrep ©FlexiPrep. Report ©violations @https://tips.fbi.gov/ Physical chemistry is the study of how matter behaves on a molecular and atomic level and how chemical reactions occur. Physical chemistry is the branch of chemistry devoted to the study of the behavior of matter at an atomic or molecular level. Based on their analyses, physical chemists may develop new theories, such as how complex structures are formed. Physical chemists often work closely with materials 2 of 6 9/22/2021 Title: Physical Chemistry- FlexiPrep scientists to research and develop potential uses for new materials. It also involves the study of the properties of substances at different scales, from the macroscopic scale which includes particles that are visible to the naked eye, to the subatomic scale involving extremely small subatomic particles such as electrons. Physical chemistry differs from other branches of chemistry because it employs the concepts and principles of physics to understand chemical systems and reactions. The different scales that this branch of chemistry deals with are described below. Macroscopic Scale 3 of 6 9/22/2021 Title: Physical Chemistry- FlexiPrep ©FlexiPrep. Report ©violations @https://tips.fbi.gov/ The macroscopic scale involves the substances that are large enough to be visible to the human eye The macroscopic scale is the length scale on which objects or phenomena are large enough to be visible with the naked eye, without magnifying optical instruments.