The·Adelie:'Lan'o:Mete'orite'i
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Meteorite in Den Polargebiefen
Literatur: chemische Untersuchungen in den Alpen. Zb1. 1) Gor h a m , E.: The salt content of some Ice Aerosolforsch. 10, 1962, 97-105. samples from Nordaustlandet, Svalbord, :r. of 6) Tarn a n n , G.: Die Bildung des Gletscher Glaciology, 3, 1957, 181-186. korns, Naturwiss. 17, 1929, 851-854. 2) Gor h a m , E.: Soluble salts in a temperate 7) Renaud, A.: A contribution to the study of glacier Tellus, 10, 1958, 496-497. the glacter-graln. J. of Glaciology, 1, 1949, 3) Renaud, A.: Nouvelle contribution ä l' Hude 328-324. du grain de glacier. IUGG-Konferenzbericht, 8) R e v eIl e, R. und S u e s S, H.: Carbon dio Brüssel 1951, 206-211. xide exehange between atmosphere and ocean 4) Junge, C. E.: Sulfur in the atmosphere. :r of and the question of anincrease of atmospheric Geophys. Res .. 65, 1960, 227-237. CO. during the past decades Tellus, 9, 1957, 5) G e 0 r g 11, H. W. und Web er, E.: Luft- 18-27. Meteorite in den Polargebiefen Von Werner Sandner, Grafing-Bahnhof '}) Gegenüber den Vertretern aller anderen tische Gebiet zu zählen sind und wieviele naturwissenschaftlichen Disziplinen ist der Fundorte bereits südlich desselben liegen. Astronom insofern benachteiligt, als er die Was die benutzten literarischen Unterlagen Objekte seines Forschens nicht im Labora betrifft, so reicht der Katalog von Leonard torium einer Untersuchung unterwerfen, bis zum Jahre 1955, während das Werk von nicht mit ihnen "experimentieren" kann. Er Krinow alle Fälle und Funde bis 1956 ent ist vielmehr auf die Kunde angewiesen, die hält. -

Cape Denison MAWSON CENTENNIAL 1911–2011, Commonwealth Bay
Cape Denison MAWSON CENTENNIAL 1911–2011, Commonwealth Bay Mawson and the Australasian Geology of Cape Denison Landforms of Cape Denison Position of Cape Denison in Gondwana Antarctic Expedition The two dominant rock-types found at Cape Denison Cape Denison is a small ice-free rocky outcrop covering Around 270 Million years ago the continents that we are orthogneiss and amphibolite. There are also minor less than one square kilometre, which emerges from The Australasian Antarctic Expedition (AAE) took place know today were part of a single ancient supercontinent occurrences of coarse grained felsic pegmatites. beneath the continental ice sheet. Stillwell (1918) reported between 1911 and 1914, and was organised and led by called Pangea. Later, Pangea split into two smaller that the continental ice sheet rises steeply behind Cape the geologist, Dr Douglas Mawson. The expedition was The Cape Denison Orthogneiss was described by Stillwell (1918) as supercontinents, Laurasia and Gondwana, and Denison reaching an altitude of ‘1000 ft in three miles and jointly funded by the Australian and British Governments coarse-grained grey quartz-feldspar layered granitic gneiss. These rock Antarctica formed part of Gondwana. with contributions received from various individuals and types are normally formed by metamorphism (changed by extreme heat 1500 ft in five and a half miles’ (approximately 300 metres and pressure) of granites. The Cape Denison Orthogneiss is found around In current reconstructions of the supercontinent Gondwana, the Cape scientific societies, including the Australasian Association to 450 metres over 8.9 kilometres). Photography by Chris Carson Cape Denison, the nearby offshore Mackellar Islands, and nearby outcrops Denison–Commonwealth Bay region was located adjacent to the coast for the Advancement of Science. -
For a Complete List Click Here
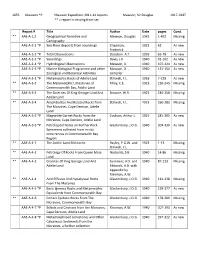
1655 Mawson *P Mawson Expedition 1911-14 reports Mawson, Sir Douglas 1917-1947 ** = report is missing from set Report # Title Author Date pages Cond. ** AAE-A-1,2 Geographical Narrative and Mawson, Douglas 1942 1-492 Missing Cartography AAE-A-2-1 *P Sea-Floor deposits from soundings Chapman, 1922 62 As new Frederick AAE-A-2-2 *P Tidal Observations Doodson. A.T. 1939 65-78 As new AAE-A-2-3 *P Soundings Davis, J.K. 1940 91-102 As new AAE-A-2-4 *P Hydrological Observations Mawson, D. 1940 107-124 As new AAE-A-2-5 *P Marine Biological Programme and other Mawson, D. , 1940 131-150 As new Zoological and Botanical Activities compiler AAE-A-3-1 *P Metamorphic Rocks of Adelie Land Stillwell, F.L. 1918 7-229 As new ** AAE-A-3-2 The Metamorphic Limestones of Tilley, C.E. 1923 230-245 Missing Commonwealth Bay, Adelie Land ** AAE-A-3-3 The Dolerites Of King George Land And Browne, W.R. 1923 246-258 Missing Adelie Land ** AAE-A-3-4 Amphibolites And Related Rocks from Stillwell, F.L. 1923 260-280 Missing The Moraines, Cape Denison, Adelie Land AAE-A-3-5 *P Magnetite Garnet Rocks from the Coulson, Arthur L. 1925 281-305 As new Moraines, Cape Denison, Adelie Land AAE-A-3-6 *P Petrological Notes on Further Rock Glastonbury, J.O.G. 1940 309-330 As new Specimens collected from in situ occurrences in Commonwealth Bay Region ** AAE-A-4-1 The Adelie Land Meteorite Bayley, P.G.W. -

Cape Denison, the Birthplace of Australian Antarctic Expeditions New Map and Poster Commemorates the Australasian Antarctic Expedition
ISSUE 104 Dec 2011 Cape Denison, the birthplace of Australian Antarctic expeditions New map and poster commemorates the Australasian Antarctic Expedition Chris Carson This year marks the 100th anniversary of the Australasian Antarctic Mawson decided that Cape Expedition (AAE, 1911–1914) which was organised and led by the Denison would be the location eminent geologist Dr (later Sir) Douglas Mawson. The objectives for the main expedition base of the AAE were the exploration and scientific investigation of the (figure 1). During the initial then largely unknown coastline and hinterland in the vicinity of landing at Cape Denison weather Adélie Land. This expedition marked the start of Australia’s long conditions were pleasant enough, engagement with Antarctica, a productive and active involvement with Mawson recording ‘the day that continues to this day. had been perfect’. However, the The expedition departed Hobart on 2 December 1911 on the true character of Commonwealth Aurora, under command of Captain John K Davis, and established Bay was soon to reveal itself with a vital support base on Macquarie Island before continuing south to katabatic winds, that drain dense Antarctica and establishing the main base. Finding a suitable site for cold air from the polar plateau a base was difficult and Mawson finally located a small rocky ice- inland behind Commonwealth free cape, later named Cape Denison after Sir Hugh Denison, one Bay, which are the most ferocious of the major expedition supporters, located within Commonwealth and persistent winds on the Bay. The bay was named for the newly federated Australian planet. Cecil Madigan, the AAE Commonwealth and, according to Charles Laserson the expedition meteorologist, recorded a mean biologist, ‘stretched in a great semicircle, bordered everywhere by wind-speed of over 71 kilometres high ice cliffs, with here and there a patch of black rock showing at per hour over nearly two years of the base’. -

Xb Ie'ian%Mlseltm
Xb1oxfitateie'ian%Mlseltm PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK 24, N.Y. NUMBER 2 I 90 SEPTEMBER IO, I964 The Meteorite and Tektite Collection of the American Museum of Natural History BY BRIAN MASON' INTRODUCTION The first meteorite received by the American Museum of Natural History was a 46-gram piece of the Searsmont chondrite, presented by G. M. Brainerd of Rockland, Maine, in 1872. For some years the col- lection grew very slowly. Hovey (1896) published the first catalogue, in which he enumerated 55 pieces representing 26 different meteorites. However, the status of the collection was radically changed in 1900 with the acquisition of the Bement collection of minerals, through the gener- osity ofJ. Pierpont Morgan. Besides some 12,000 mineral specimens, the Bement collection contained 580 meteorites, representing nearly 500 different falls and finds. This acquisition established the meteorite col- lection of this museum as one of the great collections of the world, a situation that has been maintained by more recent additions. Some of the more notable additions may be briefly noted. In 1904 three Cape York irons, brought from Greenland in 1897 by R. E. Peary, were deposited in the museum. These are known as "Ahnighito" or "The Tent," "The Woman," and "The Dog" (fig. 1). "Ahnighito," the largest of the three, is approximately 11 feet long, 7 feet high, and 6 feet thick. Various estimates of its weight, ranging from 30 to 80 tons, have been published. Thanks to the Toledo Scale Company it was mounted 1 Chairman, Department of Mineralogy, the American Museum of Natural History. -
Cape Denison Station, Adelie Land
AUSTRALASIAN i ANTARCTIC EXPEDITION 1911-14. UNDER THE LEADERSHIP OP SIR DOUGLAS AAWSON, O.B.E., B.E., D.Sc, F.R.S. SCIENTIFIC REPORTS. SERIES B. VOL. IV. METEOROLOGY. TABULATED AND REDUCED RECORDS OF THE CAPE DENISON STATION, ADELIE LAND. BY C. T. MADIGAN, M.A. (Oxon.), B.Sc, Dip. M.E. (Adel.), F.G.S.. Chief Meteorologist, Adelie Land Station. WITH TWENTY PLATES. TWENTY-FOUR FIGURES IN TEXT, AND SIXTEEN TABLES, AND AN APPENDIX BY W. E. BAS5ETT, W.E.E. PRICE: THIRTY SHILLINGS. ALrFEO JAMES KENT, GOVERNMENT PHIVTPH. PWI LIP-STREET. SYONKT.—IM». ISSUED JUNE, 1929. SERIES A—REPORTS. HON. EDITOR : PROF. SIR DOUGLAS MAWSON, KT., D.SO., B.B., University of Adelaide. VOL. PBIOI. £ 8. i I. GEOGRAPHY AND PHYSIOGRAPHY. {In preparation.) II. OCEANOGRAPHY. PART 1.—SEA-FLOOR DEPOSITS FROM SOUNDINGS— By FREDERICK CHAPMAN, ASS. Linn. Soc. (Lond.), F.R.M.S., &c, National Museum, Melb. 0 6 0 III. GEOLOGY. (Adelie Land and King George Land.) PART 1.—THE METAMORPHIG ROOKS OF ADELIE LAND— By F. L. STILLWELL, D.SC , Aust. Antarc. Ezped. Staff 2 2 0 „ 2.—THE METAMORPHIG LIMESTONES OF COMMONWEALTH BAY, ADELIE LAND. By 0. E. TILLEY, B.Sc. 0 1 6 „ 3.—THE DOLERITES OF KING GEORGE LAND AND ADELIE LAND. By W. R. BROWNE, D.SC , Lecturer, Geological Department, Sydney University 0 1 6 „ 4.—AMPHIBOLITES AND RELATED ROCKS FROM THE MORAINES, GAPE DENISON, ADELIE LAND. By F. L. STILLWELL, D.SC, AuBt. Antarc. Ezped. Staff 0 2 0 IV. GEOLOGY. {Will deal principally with Queen Mary Land.) {In preparation.) PART 1—THE ADELIE LAND METEORITE— By P. -

Download 1 File
Report of the Secretary and Financial Report of the Executive Committee of the Board of Regents Smithsonian Institution Report of the Secretary and Financial Report of the Executive Committee of the Board of Regents For the year ended June 30 1960 Smithsonian Publication 4429 U.S. GOVERNMENT PRINTING OFFICE WASHINGTON : 1960 CONTENTS Page List of officials v General statement 1 The Establishment 6 The Board of Regents q Finances 7 Visitors 7 Reports of branches of the Institution: United States National Museum 9 Bureau of American Ethnology 48 Astrophysical Observatory 83 National Collection of Fine Arts 97 Freer Gallery of Art 106 National Air Museum 119 National Zoological Park 131 Canal Zone Biological Area 172 International Exchange Service 177 National Gallery of Art 186 Report on the library 199 Report on publications 202 Other activities: Lectures 211 Bio-Sciences Information Exchange 211 Smithsonian Museum Service 212 Report of the executive committee of the Board of Regents 214 in THE SMITHSONIAN INSTITUTION June 30, 1960 Presiding Officer ex officio.—Dwight D. Eisenhower, President of the United States. Chancellor.—Eael Wabben, Chief Justice of the United States. Members of the Institution: Dwight D. Eisenhowee, President of the United States. RiCHAED M. Nixon, Vice President of the United States. Earl Wareen, Chief Justice of the United States. Christian A. Heeter, Secretary of State. RoBEET B. Andebson, Secretary of the Treasury. Thomas S. Gates, Je., Secretary of Defense. William P. Rogees, Attorney General. Abthtje E. Summebpield, Postmaster General. Feed A. Seaton, Secretary of the Interior. EzBA Taft Benson, Secretary of Agriculture. Feedeeick H. Mueller, Secretary of Commerqe. -

Geophysical Abstracts 184 January-March 1961
Geophysical Abstracts 184 January-March 1961 By JAMES W. CLARKE, DOROTHY B. VITALIANO, VIRGINIA S. NEUSCHEL, and others GEOLOGICAL SURVEY BULLETIN 1146-A Abstracts of current literature pertaining to the physics of the solid earth and to geophysical exploration UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1961 UNITED STATES DEPARTMENT OF THE INTERIOR STEWART L. UDALL, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director For sale by the Superintendent of Documents, U.S. Government Printin~ Office, Washin~ton 25, D.C. Price 40 cents (sin~e copy). Subscription price: $1.75 a year; 50 cents additional for foreign mailin~. Use of funds for printin~ this publication has been approved by the Director of the Bureau of the Bud~et (June 23, 1960). CONTENTS Page Introduction --------------------------------------------------- 1 Extent of coverage------------------------------------------ 1 List of journals -------------------------------------------- 1 Form of citation-------------------------------------------- 2 Abstracters------------------------------------------------ 2 Age determinations -------------------------------------------- 2 Cosmogony---------------------------------------------------- 23 Earth currents ------------------------------------------------ 34 Earthquakes and earthquake waves------------------------------- 37 Elasticity----------------------------------------------------- 59 Electrical exploration ------------------------------------------ 71 Electrical logging---------------------------------------------- 79 -

Origin and Significance of Antarctic Meteorites Harvey, R
Chemie der Erde · Geochemistry ·63(2003)2S.93-184 ·Geochemistry Erde der Chemie Band 63 · 2003 Heft 2 The Origin and Significance of Antarctic Meteorites Harvey, R. ..................................................... 93 Origin and Formational Environment of Noda-Tamagawa Manganese Ore, Northeast Japan: Constraints from Isotopic Studies El Rhazi, M. and Hayashi, K.-i. ......................................... 149 Mineralogy and Chemistry of Biotites from Eastern Pontide Granitoid Rocks, NE-Turkey: Some Petrological Implications for Granitoid Magmas Interdisciplinary Journal for Chemical Problems of the Geosciences and Geoecology Aydin F., Karsli, O., and Sadiklar, M. B. ................................... 163 Hinweise für Autoren ......... www.urbanfischer.de/journals/chemerd 183 Begründet 1914 von Gottlob Linck Fortgeführt von Fritz Heide Editor-in-Chief Klaus Heide, Jena 2 Indexed in Band 63 CURRENT CONTENTS, Physical, Chemical & Earth Sciences • Sciene Citation Index- Mai 2003 expanded • Chemcial Abstracts • Economic Geology • Geo Abstracts • GEOBASE • ISI Alerting Service • INIS Atomid • Deep Sea Research & Oceanographic Abstracts • GeoRef • Mineralogical Abstracts • Referativnyi Zhurnal • Research Alert • SciSearch Table of Contents now also available via e-mail by free-of-charge ToC Alert Service. Register now: http://www.urbanfischer.de/journals/chemerd URBAN & FISCHER Verlag · Jena/Germany ISSN 0009-2819 · Chem. Erde · 63(2003)2 · S. 93-184 Chem. Erde 63 (2003), 93–147 Urban & Fischer Verlag http://www.urbanfischer.de/journals/chemerd -

Research School of Earth Sciences Annual Report 2018
Research School of Earth Sciences Annual Report 2018 Detection of shear waves in the Earth’s inner core. Image credit: Thanh-Son Pham and Hrvoje Tkalčić TABLE OF CONTENTS DIRECTOR’S REVIEW OF 2018 STAFF and STUDENT LISTS Academic Staff Professional Staff Postgraduate Students Undergraduate & Postgraduate courses THESES AND AWARDS Theses Completed Staff Honours and Awards Student Honours and Awards RESEARCH GROUP ACTIVITIES Biogeochemistry Climate and Fluid Physics Earth Dynamics Experimental Petrology Geochemistry & Cosmochemistry Palaeoenvironments Seismology & Mathematical Geophysics International Ocean Discovery Program RESEARCH SUPPORT Electronics Group Engineering Workshop RESEARCH GRANTS PEER-REVIEWED PUBLICATIONS EXTERNAL COMMITTEES AND EDITORIAL BOARDS DIRECTOR’S REVIEW OF 2018 2018 has been a highly successful year for the School by many measures ranging from high quality research publications, a significant increase in awarded research funding, major changes to the School’s education program now implemented, and major awards received by current and emeritus staff. Significant changes in academic staffing occurred with Dr Callum Shakespeare being offered a tenure-track position into the Climate Fluid Physics group, and Dr Mark Kendrick being converted to a continuing position in the Geochemistry and Cosmochemistry group. Professor Stephen Cox and Professor Brad Pillans retired in mid-2018, but both continue to be highly research active. Associate Professors Andy Hogg, Penny King, and Hrvoje Tkalcic were all promoted to Level E (Professor), Dr Rhodri Davies was promoted to Level D (A/Professor) and Dr Katharine Grant to Level B (Research Fellow). New professional staff arrivals included Shanti Devi, Matt Francis, and Larisa Medenis who joined the School’s administration team in various roles. -

Australian Meteorites
AUSTRALIAN MUSEUM SCIENTIFIC PUBLICATIONS Hodge-Smith, T., 1939. Australian meteorites. Australian Museum Memoir 7: 1–84, plates i–xix. [3 July 1939]. doi:10.3853/j.0067-1967.7.1939.478 ISSN 0067-1967 Published by the Australian Museum, Sydney naturenature cultureculture discover discover AustralianAustralian Museum Museum science science is is freely freely accessible accessible online online at at www.australianmuseum.net.au/publications/www.australianmuseum.net.au/publications/ 66 CollegeCollege Street,Street, SydneySydney NSWNSW 2010,2010, AustraliaAustralia THE AUSTRALIAN MUSEUM, SYDNEY· MEMOIR VII. AUSTRALIAN METEORITES By T. HODGE-SMITH. PUBLISHED BY ORDER OF THE TRUSTEES. Charles Anderson, M.A., D.Sc., C.M.Z.S., Director. Sydney, July 3. 1939. CONTENTS. PAGE•. Introduction 1 General 1 Classification 3 Australian Falls 9 Map showing sites of Falls in Australia and Tasmania 10-11 Table showing distribution of Falls in Australia, and those represented in the collection of the Australian Museum .. 28 Map showing Distribution of the "Cranbourne" Masses 30 Map showing Distribution of the "Bingara" Masses 31 Historical 34 Meteorite Craters 36 Mineralogy- Native Elements 39 Structure of the Nickel-iron Alloys 41 Diagram illustrating the hypothesis of Osmond-Roozeboom 42 Carbides 45 Sulphides 45 Phosphides 46 Chlorides 48 Oxides 48 Carbonates 49 Silicates 49 Petrology 52 Chemistry- Siderites 53 Siderolites 57 Aerolites 59 Norms calculated from analyses 64 Tektites 65 Map of Australia showing Distribution of Australites 66 Literature 70 Appendix. List of foreign meteorites in the collection of the Australian Museum 80 Explanation of Plates 82 Plates I-XIX End. AUSTRALIAN METEORITES. By T. HODGE-SMITH, Mineralogist, The Australian Museum. -

Macouarie Island Station
AUSTRALASIAN ANTARCTIC EXPEDITION 1911-14 UNDER THE LEADERSHIP OF SIR DOUGLAb A\AWSON, O.B.E., DSc. B.E., P.R.S. SCIENTIFIC REPORTS. SERIES B. VOL. III. METEOROLOGY. TABULATED AND REDUCED RECORDS ' OF THE MACOUARIE ISLAND STATION RECORDERS: G. F. A1NSWORTH, H. POWER, AND A. C. TULLOCH, Officers of the Commonwealth Meteorological Service assigned by Mr. H. A Hunt, Director, to conduct the observations. REDUCTION AND TABULATION OF DATA. By direction of Mr. H. A. Hunt, and under Superintendence or B. W. NEWMAN, Member of the Staff of the Commonwealth Meteorological Bureau. WITH FOUR PLATES, TWO FIGURES IN TEXT, AND THIRTEEN TABLES, • PRICE: FORTY SHILLINGS. INTPD RV ALFRED JAMES KE.NT. GOVERNMENT PBINTER.PHllLIP-t-nWKT. SYDNEY 1029 ISSUED JUNE,: 1929. SERIES A—REPORTS. HON. EDITOR: PROF. SIR DOUGLAS MAWSON", KT., D.SC., B.E., University of Adelaide. VOL. PRIC». £ s. d. I. GEOGRAPHY AND PHYSIOGRAPHY. (In preparation.) II. OCEANOGRAPHY. PART 1.—SEA-FLOOR DEPOSITS FROM SOUNDINGS— By FREDERICK CHAPMAN, ASS. Linn. SOC. (Lond.), F.R.M.S., <fcc, National Museum, Melb. 0 5 0 III. GEOLOGY. (Adelie Land and King George Land.) PART 1.—THE METAMORPHIC ROCKS OF ADELIE LAND— By F. L, STILLWELL, D.SC, Aust. Antarc. Exped. Stafl 2 2 0 „ 2.—THE METAMORPHIC LIMESTONES OF COMMONWEALTH BAY, ADELIE LAND. By C. E. TILLEY, B.Sc 0 I' S ., 3.—THE DOLERITES OF KING GEORGE LAND AND ADELIE LAND. By W. R. BROWNE, D.SC, Lecturer, Geological Department, Sydney University 0 I , G , 4.—AMPHIBOLITES AND RELATED ROCKS FROM THE MORAINES, CAPE DENISON, ADELIE LAND. By F.