Short Communications High-Performance Thin-Layer Chromatographic Detection of Profenofos in Biological Materials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Interactive Effects of Imidacloprid, Profenofos and Carbosulfan at Low Concentrations on Homeostasis and Haematological Indices in Male Albino Rats
INTERACTIVE EFFECTS OF IMIDACLOPRID, PROFENOFOS AND CARBOSULFAN AT LOW CONCENTRATIONS ON HOMEOSTASIS AND HAEMATOLOGICAL INDICES IN MALE ALBINO RATS Kandil, M.A.*; El-Kashoury, A.A.**; El-Said, M.M.** and El-Herrawy, M.A.** J. Egypt. Soc. Toxicol. (Vol. 35: 69-78 July 2006) * Economic Entomology & Pesticides Dept., Fac. of Agric., Cairo University. WWW.estoxicology.org ** Mammalian & Aquatic Toxicology Dept., Central Agricultural Pesticides Lab., Agric. Res. Center, Dokki-Giza, Egypt. ABSTRACT Toxicity data with single pesticides to test animals are far more abundant than with mixtures (Flipo et al., 1992). Consequently, these data cannot be used directly to predict the effect of pesticide combinations. Three pesticides; imidacloprid, profenofos and carbosulfan, administered to rats per OS at low level dose equal 1/30 LD50 for each insecticide, which represent 111, 70 and 43 ppm, respectively on homeostasis status and haematological indices (El-Kashory & El-Said, 2001), were selected to explore their combined action of subchronic exposure studies for 90 days in adult male albino rats. Homeostasis-related parameters such as; aldosterone (Ald.), sodium ions (Na+), potassium ions (K+), total chloride ions (T.Cl-) levels, pH value and haematological indices were examined in rats after an administration with different insecticide combinations. Moreover, after withdrawal the pesticide combinations for 30 days, as a recovery period, the above mentioned parameters were evaluated, in comparison with the control group. Results showed that, pesticide combination imidacloprid/profenofos (I + P) induced significant decrease in Na+ and T.Cl- ions levels and significant increase in pH value. While, it did not alter both Ald. and K+ ions levels. -

Table II. EPCRA Section 313 Chemical List for Reporting Year 2017 (Including Toxic Chemical Categories)
Table II. EPCRA Section 313 Chemical List For Reporting Year 2017 (including Toxic Chemical Categories) Individually listed EPCRA Section 313 chemicals with CAS numbers are arranged alphabetically starting on page II-3. Following the alphabetical list, the EPCRA Section 313 chemicals are arranged in CAS number order. Covered chemical categories follow. Note: Chemicals may be added to or deleted from the list. The Emergency Planning and Community Right-to-Know Call Center or the TRI-Listed Chemicals website will provide up-to-date information on the status of these changes. See section B.3.c of the instructions for more information on the de minimis % limits listed below. There are no de minimis levels for PBT chemicals since the de minimis exemption is not available for these chemicals (an asterisk appears where a de minimis limit would otherwise appear in Table II). However, for purposes of the supplier notification requirement only, such limits are provided in Appendix C. Chemical Qualifiers Certain EPCRA Section 313 chemicals listed in Table II have parenthetic “qualifiers.” These qualifiers indicate that these EPCRA Section 313 chemicals are subject to the section 313 reporting requirements if manufactured, processed, or otherwise used in a specific form or when a certain activity is performed. An EPCRA Section 313 chemical that is listed without a qualifier is subject to reporting in all forms in which it is manufactured, processed, and otherwise used. The following chemicals are reportable only if they are manufactured, processed, or otherwise used in the specific form(s) listed below: Chemical/ Chemical Category CAS Number Qualifier Aluminum (fume or dust) 7429-90-5 Only if it is a fume or dust form. -

744 Hydrolysis of Chiral Organophosphorus Compounds By
[Frontiers in Bioscience, Landmark, 26, 744-770, Jan 1, 2021] Hydrolysis of chiral organophosphorus compounds by phosphotriesterases and mammalian paraoxonase-1 Antonio Monroy-Noyola1, Damianys Almenares-Lopez2, Eugenio Vilanova Gisbert3 1Laboratorio de Neuroproteccion, Facultad de Farmacia, Universidad Autonoma del Estado de Morelos, Morelos, Mexico, 2Division de Ciencias Basicas e Ingenierias, Universidad Popular de la Chontalpa, H. Cardenas, Tabasco, Mexico, 3Instituto de Bioingenieria, Universidad Miguel Hernandez, Elche, Alicante, Spain TABLE OF CONTENTS 1. Abstract 2. Introduction 2.1. Organophosphorus compounds (OPs) and their toxicity 2.2. Metabolism and treatment of OP intoxication 2.3. Chiral OPs 3. Stereoselective hydrolysis 3.1. Stereoselective hydrolysis determines the toxicity of chiral compounds 3.2. Hydrolysis of nerve agents by PTEs 3.2.1. Hydrolysis of V-type agents 3.3. PON1, a protein restricted in its ability to hydrolyze chiral OPs 3.4. Toxicity and stereoselective hydrolysis of OPs in animal tissues 3.4.1. The calcium-dependent stereoselective activity of OPs associated with PON1 3.4.2. Stereoselective hydrolysis commercial OPs pesticides by alloforms of PON1 Q192R 3.4.3. PON1, an enzyme that stereoselectively hydrolyzes OP nerve agents 3.4.4. PON1 recombinants and stereoselective hydrolysis of OP nerve agents 3.5. The activity of PTEs in birds 4. Conclusions 5. Acknowledgments 6. References 1. ABSTRACT Some organophosphorus compounds interaction of the racemic OPs with these B- (OPs), which are used in the manufacturing of esterases (AChE and NTE) and such interactions insecticides and nerve agents, are racemic mixtures have been studied in vivo, ex vivo and in vitro, using with at least one chiral center with a phosphorus stereoselective hydrolysis by A-esterases or atom. -

Organophosphate Insecticides
CHAPTER 4 HIGHLIGHTS Organophosphate Insecticides Acts through phosphorylation of the acetylcholinesterase enzyme Since the removal of organochlorine insecticides from use, organophosphate at nerve endings insecticides have become the most widely used insecticides available today. More Absorbed by inhalation, than forty of them are currently registered for use and all run the risk of acute ingestion, and skin and subacute toxicity. Organophosphates are used in agriculture, in the home, penetration in gardens, and in veterinary practice. All apparently share a common mecha- Muscarinic, nicotinic & CNS nism of cholinesterase inhibition and can cause similar symptoms. Because they effects share this mechanism, exposure to the same organophosphate by multiple routes or to multiple organophosphates by multiple routes can lead to serious additive Signs and Symptoms: toxicity. It is important to understand, however, that there is a wide range of Headache, hypersecretion, toxicity in these agents and wide variation in cutaneous absorption, making muscle twitching, nausea, specific identification and management quite important. diarrhea Respiratory depression, seizures, loss of consciousness Toxicology Miosis is often a helpful Organophosphates poison insects and mammals primarily by phosphory- diagnostic sign lation of the acetylcholinesterase enzyme (AChE) at nerve endings. The result is a loss of available AChE so that the effector organ becomes overstimulated by Treatment: the excess acetylcholine (ACh, the impulse-transmitting substance) in the nerve Clear airway, improve tissue ending. The enzyme is critical to normal control of nerve impulse transmission oxygenation from nerve fibers to smooth and skeletal muscle cells, glandular cells, and Administer atropine sulfate autonomic ganglia, as well as within the central nervous system (CNS). -

Environmental Health Criteria 63 ORGANOPHOSPHORUS
Environmental Health Criteria 63 ORGANOPHOSPHORUS INSECTICIDES: A GENERAL INTRODUCTION Please note that the layout and pagination of this web version are not identical with the printed version. Organophophorus insecticides: a general introduction (EHC 63, 1986) INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY ENVIRONMENTAL HEALTH CRITERIA 63 ORGANOPHOSPHORUS INSECTICIDES: A GENERAL INTRODUCTION This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization World Health Orgnization Geneva, 1986 The International Programme on Chemical Safety (IPCS) is a joint venture of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. The main objective of the IPCS is to carry out and disseminate evaluations of the effects of chemicals on human health and the quality of the environment. Supporting activities include the development of epidemiological, experimental laboratory, and risk-assessment methods that could produce internationally comparable results, and the development of manpower in the field of toxicology. Other activities carried out by the IPCS include the development of know-how for coping with chemical accidents, coordination -

Pesticides Contamination of Cereals and Legumes: Monitoring of Samples Marketed in Italy As a Contribution to Risk Assessment
applied sciences Article Pesticides Contamination of Cereals and Legumes: Monitoring of Samples Marketed in Italy as a Contribution to Risk Assessment Valeria Nardelli 1, Valeria D’Amico 1, Mariateresa Ingegno 1 , Ines Della Rovere 1, Marco Iammarino 1,* , Francesco Casamassima 1, Anna Calitri 1, Donatella Nardiello 2 , Donghao Li 3 and Maurizio Quinto 2,3,* 1 Istituto Zooprofilattico Sperimentale della Puglia e della Basilicata, Via Manfredonia 20, 71121 Foggia, Italy; [email protected] (V.N.); [email protected] (V.D.); [email protected] (M.I.); [email protected] (I.D.R.); [email protected] (F.C.); [email protected] (A.C.) 2 Dipartimento di Scienze Agrarie, Alimenti, Risorse Naturali e Ingegneria—Università degli Studi di Foggia, Via Napoli, 25, 71122 Foggia, Italy; [email protected] 3 Department of Chemistry, Yanbian University, Park Road 977, Yanji 133002, China; [email protected] * Correspondence: [email protected] (M.I.); [email protected] (M.Q.) Featured Application: This work offers a contribution to risk assessment regarding the levels of 37 pesticides in cereal and legume samples commercialized in Italy during the last years. It is well-known that prolonged exposure to pesticides can increase the risk of cardiovascular and respiratory disease, other than promoting cancer diseases. Thus, the World Health Organization and the European Food Safety Authority ask for monitoring of the levels of such substances, Citation: Nardelli, V.; D’Amico, V.; especially in vegetables, continuously, to have availability updated and detailed data on this Ingegno, M.; Della Rovere, I.; Iammarino, M.; Casamassima, F.; type of food contamination. -

Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 Theinternational Programme on Chemical Safety (IPCS) Was Established in 1980
The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 cation Hazard of Pesticides by and Guidelines to Classi The WHO Recommended Classi The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 TheInternational Programme on Chemical Safety (IPCS) was established in 1980. The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. This publication was developed in the IOMC context. The contents do not necessarily reflect the views or stated policies of individual IOMC Participating Organizations. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase international coordination in the field of chemical safety. The Participating Organizations are: FAO, ILO, UNDP, UNEP, UNIDO, UNITAR, WHO, World Bank and OECD. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment. WHO recommended classification of pesticides by hazard and guidelines to classification, 2019 edition ISBN 978-92-4-000566-2 (electronic version) ISBN 978-92-4-000567-9 (print version) ISSN 1684-1042 © World Health Organization 2020 Some rights reserved. -
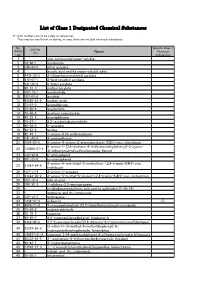
List of Class 1 Designated Chemical Substances
List of Class 1 Designated Chemical Substances *1:CAS numbers are to be solely as references. They may be insufficient or lacking, in case there are multiple chemical substances. No. Specific Class 1 CAS No. (PRTR Chemical (*1) Name Law) Substances 1 - zinc compounds(water-soluble) 2 79-06-1 acrylamide 3 140-88-5 ethyl acrylate 4 - acrylic acid and its water-soluble salts 5 2439-35-2 2-(dimethylamino)ethyl acrylate 6 818-61-1 2-hydroxyethyl acrylate 7 141-32-2 n-butyl acrylate 8 96-33-3 methyl acrylate 9 107-13-1 acrylonitrile 10 107-02-8 acrolein 11 26628-22-8 sodium azide 12 75-07-0 acetaldehyde 13 75-05-8 acetonitrile 14 75-86-5 acetone cyanohydrin 15 83-32-9 acenaphthene 16 78-67-1 2,2'-azobisisobutyronitrile 17 90-04-0 o-anisidine 18 62-53-3 aniline 19 82-45-1 1-amino-9,10-anthraquinone 20 141-43-5 2-aminoethanol 21 1698-60-8 5-amino-4-chloro-2-phenylpyridazin-3(2H)-one; chloridazon 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-3-cyano- 22 120068-37-3 4[(trifluoromethyl)sulfinyl]pyrazole; fipronil 23 123-30-8 p-aminophenol 24 591-27-5 m-aminophenol 4-amino-6-tert-butyl-3-methylthio-1,2,4-triazin-5(4H)-one; 25 21087-64-9 metribuzin 26 107-11-9 3-amino-1-propene 27 41394-05-2 4-amino-3-methyl-6-phenyl-1,2,4-triazin-5(4H)-one; metamitron 28 107-18-6 allyl alcohol 29 106-92-3 1-allyloxy-2,3-epoxypropane 30 - n-alkylbenzenesulfonic acid and its salts(alkyl C=10-14) 31 - antimony and its compounds 32 120-12-7 anthracene 33 1332-21-4 asbestos ○ 34 4098-71-9 3-isocyanatomethyl-3,5,5-trimethylcyclohexyl isocyanate 35 78-84-2 isobutyraldehyde -

United States Patent (19) 11 Patent Number: 5,703,064 Yokoi Et Al
US005703064A United States Patent (19) 11 Patent Number: 5,703,064 Yokoi et al. 45) Date of Patent: Dec. 30, 1997 54 PESTICIDAL COMBINATIONS FOREIGN PATENT DOCUMENTS 75 Inventors: Shinji Yokoi; Akira Nishida, both of 0 196524 10/1986 European Pat. Of.. Shiga-ken; Tokio Obata; Kouichi Golka, both of Ube, all of Japan OTHER PUBLICATIONS 73) Assignees: Sankyo Company, Limited, Tokyo; Worthing et al, The Pesticide Manual, 9th Ed. (1991), pp. Ube industries Ltd., Ube, both of 747 and 748. Japan L.C. Gaughan et al., "Pesticide interactions: effects of orga nophosphorus pesticides on the metabolism, toxicity, and 21 Appl. No.: 405,795 persistence of selected pyrethroid insecticides". Chemical Abstracts, vol. 94, No. 9, 1981, No. 59740k of Pestic. 22 Filed: Mar 16, 1995 Biochem. Physio., vol. 14, No. 1, 1980, pp. 81-85. 30 Foreign Application Priority Data I. Ishaaya et al., "Cypermethrin synergism by pyrethroid esterase inhibitors in adults of the whitefly Bemisia tabaci". Mar 16, 1994 JP Japan ............................ HE6045.405 Chemical Abstracts, vol. 107, No. 9, 1987, No. 72818y of (51) Int. Cl................. A01N 43/54; A01N 57/00 Pestic Biochem. Physiol., vol. 28, No. 2, 1987, pp. 155-162. 52 U.S. C. ......................................... 51480; 514/256 (58) Field of Search ..................................... 514/80, 256 Primary Examiner Allen J. Robinson Attorney, Agent, or Firm-Frishauf, Holtz, Goodman, 56 References Cited Langer & Chick, P.C. U.S. PATENT DOCUMENTS 57 ABSTRACT 4,374,833 2/1983 Badmin et al. ...................... 424/225 Combinations of the known compound pyrimidifen with 4,845,097 7/1989 Matsumoto et al... 514/234.2 phosphorus-containing pesticides have a synergistic pesti 4,935,516 6/1990 Ataka et al. -

Covalent Binding of the Organophosphate Insecticide Profenofos to Tyrosine on Α- and Β-Tubulin Proteins
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Chemosphere Manuscript Author . Author manuscript; Manuscript Author available in PMC 2019 May 01. Published in final edited form as: Chemosphere. 2018 May ; 199: 154–159. doi:10.1016/j.chemosphere.2018.02.003. Covalent Binding of the Organophosphate Insecticide Profenofos to Tyrosine on α- and β-Tubulin Proteins Shaogang Chua, Margaret R. Bakerb, Gladys Leongb, Robert J. Letchera, and Qing X. Lib,* aEcotoxicology and Wildlife Health Division, Wildlife and Landscape Science Directorate, National Wildlife Research Centre, Environment and Climate Change Canada, 1125 Colonel By Dr., Carleton University, Ottawa, ON, Canada, K1A 0H3 bDepartment of Molecular Biosciences and Bioengineering, University of Hawaii at Manoa, 1955 East West Road, Honolulu, HI 96822, USA Abstract Organophosphorus (OP) compounds can bind covalently to many types of proteins and form protein adducts. These protein adducts can indicate the exposure to and neurotoxicity of OPs. In the present work, we studied adduction of tubulin with the OP insecticide profenofos in vitro and optimized the method for detection of adducted peptides. Porcine tubulin was incubated with profenofos and was then digested with trypsin, followed by mass spectrometry identification of the profenofos-modified tubulin and binding sites. With solvent-assisted digestion (80% acetonitrile in digestion solution), the protein was digested for peptide identifications, especially for some peptides with low mass. The MALDI-TOF and LC-ESI-TOF analysis results showed that profenofos bound covalently to Tyr83 in porcine α-tubulin (TGTY*83R) and to Tyr281 in porcine β-tubulin (GSQQY*281R) with a mass increase of 166.02 Da from the original peptide fragments of porcine tubulin proteins. -

NMP-Free Formulations of Neonicotinoids
(19) & (11) EP 2 266 400 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 29.12.2010 Bulletin 2010/52 A01N 43/40 (2006.01) A01N 43/86 (2006.01) A01N 47/40 (2006.01) A01N 51/00 (2006.01) (2006.01) (2006.01) (21) Application number: 09305544.0 A01P 7/00 A01N 25/02 (22) Date of filing: 15.06.2009 (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Gasse, Jean-Jacques HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL 27600 Saint-Aubin-Sur-Gaillon (FR) PT RO SE SI SK TR • Duchamp, Guillaume Designated Extension States: 92230 Gennevilliers (FR) AL BA RS • Cantero, Maria 92230 Gennevilliers (FR) (71) Applicant: NUFARM 92233 Gennevelliers (FR) (74) Representative: Cabinet Plasseraud 52, rue de la Victoire 75440 Paris Cedex 09 (FR) (54) NMP-free formulations of neonicotinoids (57) The invention relates to NMP-free liquid formulation comprising at least one nicotinoid and at least one aprotic polar component selected from the group comprising the compounds of formula I, II or III below, and mixtures thereof, wherein R1 and R2 independently represent H or an alkyl group having less than 5 carbons, preferably a methyl group, and n represents an integer ranging from 0 to 5, and to their applications. EP 2 266 400 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 266 400 A1 Description Technical Field of the invention 5 [0001] The invention relates to novel liquid formulations of neonicotinoids and to their use for treating plants, for protecting plants from pests and/or for controlling pests infestation. -

A Practical Method for Rapid Screening and Quantitative Analysis of 130 Pesticide
Electronic Supplementary Material (ESI) for Analytical Methods. This journal is © The Royal Society of Chemistry 2019 A practical method for rapid screening and quantitative analysis of 130 pesticide residues in herbal medicines based on Kovats retention index principle: An exemplary study using Panacis quinquefolii radix Peng Tan, Xi-chuan Wei, Hai-zhu Zhang, Ming Niu, Ding-kun Zhang Fig. S1 Typical TIC chromatograms of n-alkanes C9~C33 mixed standard solution Tab. S1 Monitoring ion pairs and collision energy of 130 pesticides Collision Collision Number Pesticide name m/z 1 m/z 2 CAS Number energy (V) energy (V) 1 Dichlorvos 109.0>79.0 8 185.0>93.0 14 62-73-7 2 Dichlobenil 170.9>136.0 14 170.9>100.0 24 1194-65-6 3 Mevinphos-1 127.0>109.0 12 192.0>127.0 12 7786-34-7 3 Mevinphos-2 127.0>109.0 12 192.0>127.0 12 7786-34-7 4 Butylate 156.1>57.0 10 174.1>146.1 6 2008-41-5 5 Molinate 126.1>55.0 14 187.1>126.1 6 2212-67-1 6 Tecnazene 260.9>202.9 14 202.9>142.9 22 117-18-0 7 Omethoate 156.0>110.0 8 110.0>79.0 10 1113-02-6 8 Propachlor 120.0>77.0 20 176.1>57.0 8 1918-16-7 9 Chlorethoxyfos 153.0>97.0 12 153.0>125.0 4 54593-83-8 10 Diphenylamine 169.1>66.0 24 167.1>139.1 28 122-39-4 11 Ethoprophos 200.0>158.0 6 158.0>97.0 18 13194-48-4 12 Chlorpropham 127.1>65.0 22 213.1>171.1 6 101-21-3 13 Trifluralin 306.1>264.1 8 264.1>160.1 18 1582-09-8 14 Dicrotophos 127.1>109.0 12 127.1>95.0 18 141-66-2 15 Monocrotophos 127.1>109.0 12 127.1>95.0 16 6923-22-4 16 Flumethrin 187.0>159.0 10 216.0>187.0 10 69770-45-2 17 Phorate 260.0>75.0 8 231.0>129.0 24 298-02-2