Functional Connectivity Alterations of the Temporal Lobe and Hippocampus in Semantic Dementia and Alzheimer's Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Functional Connectivity of the Angular Gyrus in Normal Reading and Dyslexia (Positron-Emission Tomography͞human͞brain͞regional͞cerebral)
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 8939–8944, July 1998 Neurobiology Functional connectivity of the angular gyrus in normal reading and dyslexia (positron-emission tomographyyhumanybrainyregionalycerebral) B. HORWITZ*†,J.M.RUMSEY‡, AND B. C. DONOHUE‡ *Laboratory of Neurosciences, National Institute on Aging, and ‡Child Psychiatry Branch, National Institute of Mental Health, National Institutes of Health, Bethesda, MD 20892 Communicated by Robert H. Wurtz, National Eye Institute, Bethesda, MD, May 14, 1998 (received for review January 19, 1998) ABSTRACT The classic neurologic model for reading, not functionally connected during a specific task. On the other based on studies of patients with acquired alexia, hypothesizes hand, if rCBF in two regions is correlated, these regions need functional linkages between the angular gyrus in the left not be anatomically linked; their activities may be correlated, hemisphere and visual association areas in the occipital and for example, because both receive inputs from a third area (for temporal lobes. The angular gyrus also is thought to have more discussion about these connectivity concepts, see refs. 8, functional links with posterior language areas (e.g., Wer- 10, and 11). nicke’s area), because it is presumed to be involved in mapping Based on lesion studies in many patients with alexia, it has visually presented inputs onto linguistic representations. Us- been proposed that the posterior portion of the neural network ing positron emission tomography , we demonstrate in normal mediating reading in the left cerebral hemisphere involves men that regional cerebral blood flow in the left angular gyrus functional links between the angular gyrus and extrastriate shows strong within-task, across-subjects correlations (i.e., areas in occipital and temporal cortex associated with the functional connectivity) with regional cerebral blood flow in visual processing of letter and word-like stimuli (12–14). -

Autism and the Social Brain a Visual Tour
Autism and the Social Brain A Visual Tour Charles Cartwright, M.D. Director YAI Autism Center “Social cognition refers to the fundamental abilities to perceive, categorize, remember, analyze, reason with, and behave toward other conspecifics” Pelphrey and Carter, 2008 Abbreviation Structure AMY Amygdala EBA Extrastriate body area STS Superior temporal sulcus TPJ Temporal parietal junction FFG Fusiform gyrus OFC Orbital frontal gyrus mPFC Medial prefrontal cortex IFG Inferior frontal Pelphrey and Carter 2008 gyrus Building Upon Structures and Function Pelphrey and Carter 2008 Neuroimaging technologies • Explosion of neuroimaging technology – Faster, more powerful magnets – Better resolution images showing thinner slices of brain • Visualize the brain in action • Explore relationship between brain structure, function, and human behavior. • Help identify what changes unfold in brain disorders like autism Neuroimaging in ASDs • No reliable neuroimaging marker • Imaging not recommended as part of routine work- up • Neuroimaging research can provide clues to brain structure and function and to neurodevelopmental origins Structural Brain Imaging • MRI and CT • Allows for examination of structure of the brain • Identifies abnormalities in different areas of brain • Helps understand patterns of brain development over time MRI • Magnetic Resonance Imaging • Uses magnetic fields and radio waves to produce high-quality two- or three dimensional images of brain structures • Large cylindrical magnet creates magnetic field around head; radio waves are -

Processing Emotional Pictures and Words: Effects of Valence and Arousal
Cognitive, Affective, & Behavioral Neuroscience 2006, 6 (2), 110-126 Processing emotional pictures and words: Effects of valence and arousal ELIZABETH A. KENSINGER and DANIEL L. SCHACTER Boston College, Chestnut Hill, Massachusetts and Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, Massachusetts There is considerable debate regarding the extent to which limbic regions respond differentially to items with different valences (positive or negative) or to different stimulus types (pictures or words). In the present event-related fMRI study, 21 participants viewed words and pictures that were neutral, negative, or positive. Negative and positive items were equated on arousal. The participants rated each item for whether it depicted or described something animate or inanimate or something common or uncommon. For both pictures and words, the amygdala, dorsomedial prefrontal cortex (PFC), and ventromedial PFC responded equally to all high-arousal items, regardless of valence. Laterality effects in the amygdala were based on the stimulus type (word left, picture bilateral). Valence effects were most apparent when the individuals processed pictures, and the results revealed a lateral/medial distinction within the PFC: The lateral PFC responded differentially to negative items, whereas the medial PFC was more engaged during the processing of positive pictures. In our daily lives, we experience many events that trig- son & Irwin, 1999). In most of the studies demonstrating ger an emotional response: We receive a compliment, wit- this preferential amygdala response, however, the fear-re- ness a car crash, or watch children playing in a park. One lated stimuli were more arousing than the other types of widely accepted framework used to classify these diverse stimuli. -

Eye-Movement Intervention Enhances Extinction Via Amygdala Deactivation
This Accepted Manuscript has not been copyedited and formatted. The final version may differ from this version. Research Articles: Behavioral/Cognitive Eye-movement intervention enhances extinction via amygdala deactivation Lycia D. de Voogd1, Jonathan W. Kanen1,3, David A. Neville1, Karin Roelofs1,2, Guillén Fernández1 and Erno J. Hermans1 1Donders Institute for Brain, Cognition and Behaviour, Radboud University and Radboud University Medical Center, 6500 HB, Nijmegen, The Netherlands 2Behavioural Science Institute, Radboud University, Nijmegen, The Netherlands 3Behavioural and Clinical Neuroscience Institute, Department of Psychology, University of Cambridge, Cambridge CB2 3EB, United Kingdom DOI: 10.1523/JNEUROSCI.0703-18.2018 Received: 14 March 2018 Revised: 10 August 2018 Accepted: 16 August 2018 Published: 4 September 2018 Author contributions: L.D.d.V., K.R., G.F., and E.J.H. designed research; L.D.d.V. and J.K. performed research; L.D.d.V. and E.J.H. contributed unpublished reagents/analytic tools; L.D.d.V. analyzed data; L.D.d.V. wrote the first draft of the paper; L.D.d.V., J.K., D.A.N., K.R., G.F., and E.J.H. edited the paper; L.D.d.V. and E.J.H. wrote the paper. Conflict of Interest: The authors declare no competing financial interests. Correspondence to: Lycia D. de Voogd, Donders Institute for Brain, Cognition and Behaviour (Radboudumc), P.O. Box 9101, 6500 HB Nijmegen, The Netherlands, Ph: +31 (0)24 36 10878, Fx: +31 (0)24 36 10989, E-mail: [email protected] Cite as: J. Neurosci ; 10.1523/JNEUROSCI.0703-18.2018 Alerts: Sign up at www.jneurosci.org/cgi/alerts to receive customized email alerts when the fully formatted version of this article is published. -

The Effects of Pharmacological Opioid Blockade on Neural Measures of Drug Cue-Reactivity in Humans
Neuropsychopharmacology (2016) 41, 2872–2881 © 2016 American College of Neuropsychopharmacology. All rights reserved 0893-133X/16 www.neuropsychopharmacology.org The Effects of Pharmacological Opioid Blockade on Neural Measures of Drug Cue-Reactivity in Humans 1 2 ,1,2 Kelly E Courtney , Dara G Ghahremani and Lara A Ray* 1 2 Department of Psychology, University of California, Los Angeles, CA, USA; Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, CA, USA Interactions between dopaminergic and opioidergic systems have been implicated in the reinforcing properties of drugs of abuse. The present study investigated the effects of opioid blockade, via naltrexone, on functional magnetic resonance imaging (fMRI) measures during methamphetamine cue-reactivity to elucidate the role of endogenous opioids in the neural systems underlying drug craving. To investigate = = = this question, non-treatment seeking individuals with methamphetamine use disorder (N 23; 74% male, mean age 34.70 (SD 8.95)) were recruited for a randomized, placebo controlled, within-subject design and underwent a visual methamphetamine cue-reactivity task during two blood-oxygen-level dependent (BOLD) fMRI sessions following 3 days of naltrexone (50 mg) and matched time for placebo. fMRI analyses tested naltrexone-induced differences in BOLD activation and functional connectivity during cue processing. The results showed that naltrexone administration reduced cue-reactivity in sensorimotor regions and related to altered functional connectivity of dorsal striatum, ventral tegmental area, and precuneus with frontal, visual, sensory, and motor-related regions. Naltrexone also weakened the associations between subjective craving and precuneus functional connectivity with sensorimotor regions and strengthened the associations between subjective craving and dorsal striatum and precuneus connectivity with frontal regions. -

Normal Cortical Anatomy
Normal Cortical Anatomy MGH Massachusetts General Hospital Harvard Medical School NORMAL CORTICAL ANATOMY • Sagittal • Axial • Coronal • The Central Sulcus NP/MGH Sagittal Neuroanatomy NP/MGH Cingulate sulcus Superior frontal gyrus Marginal ramus of Cingulate sulcus Cingulate gyrus Paracentral lobule Superior parietal lobule Parietooccipital sulcus Cuneus Calcarine sulcus Lingual gyrus Subcallosal gyrus Gyrus rectus Fastigium, fourth ventricle NP/MGH Superior frontal gyrus Cingulate sulcus Precentral gyrus Marginal ramus of Cingulate gyrus Central sulcus Cingulate sulcus Superior parietal lobule Precuneus Parietooccipital sulcus Cuneus Calcarine sulcus Frontomarginal gyrus Lingual gyrus Caudothallamic groove Gyrus rectus NP/MGH Precentral sulcus Central sulcus Superior frontal gyrus Marginal ramus of Corona radiata Cingulate sulcus Superior parietal lobule Precuneus Parietooccipital sulcus Calcarine sulcus Inferior occipital gyrus Lingual gyrus NP/MGH Central sulcus Superior parietal lobule Parietooccipital sulcus Frontopolar gyrus Frontomarginal gyrus Superior occipital gyrus Middle occipital gyrus Medial orbital gyrus Lingual gyrus Posterior orbital gyrus Inferior occipital gyrus Inferior temporal gyrus Temporal horn, lateral ventricle NP/MGH Central sulcus Superior Temporal gyrus Middle Temporal gyrus Inferior Temporal gyrus NP/MGH Central sulcus Superior parietal gyrus Inferior frontal gyrus Frontomarginal gyrus Anterior orbital gyrus Superior occipital gyrus Middle occipital Posterior orbital gyrus gyrus Superior Temporal gyrus Inferior -

Acute and Long-Lasting Cortical Thickness Changes Following Intensive first- Person Action Videogame Practice T
Behavioural Brain Research 353 (2018) 62–73 Contents lists available at ScienceDirect Behavioural Brain Research journal homepage: www.elsevier.com/locate/bbr Research report Acute and long-lasting cortical thickness changes following intensive first- person action videogame practice T Davide Momia,1, Carmelo Smeraldaa,1, Giulia Sprugnolia, Salvatore Ferronea, Simone Rossia,b,c,d, ⁎ Alessandro Rossia,d, Giorgio Di Lorenzoe, Emiliano Santarnecchia,f, a Brain Investigation & Neuromodulation Lab, Department of Medicine, Surgery and Neuroscience, Neurology and Clinical Neurophysiology Section, University of Siena, Italy b Siena Robotics and Systems Lab (SIRS-Lab), Engineering and Mathematics Department, University of Siena, Italy c Human Physiology Section, Department of Medicine, Surgery and Neuroscience, University of Siena, Siena, Italy d Department of Medicine, Surgery and Neuroscience, University of Siena, Siena, Italy e Laboratory of Psychophysiology, Chair of Psychiatry, Department of Systems Medicine, University of Rome “Tor Vergata”, Rome, Italy f Berenson-Allen Center for Non-Invasive Brain Stimulation, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA ARTICLE INFO ABSTRACT Keywords: Recent evidence shows how an extensive gaming experience might positively impact cognitive and perceptual Cortical thickness functioning, leading to brain structural changes observed in cross-sectional studies. Importantly, changes seem FPS to be game-specific, reflecting gameplay styles and therefore opening to the possibility of tailoring videogames Action videogame according to rehabilitation and enhancement purposes. However, whether if such brain effects can be induced Brain plasticity even with limited gaming experience, and whether if they can outlast the gaming period, is still unknown. Here MRI we quantified both cognitive and grey matter thickness changes following 15 daily gaming sessions based on a Attention modified version of a 3D first-person shooter (FPS) played in laboratory settings. -

Cortical Regions Involved in Eye Movements, Shifts of Attention, and Gaze Perception
᭜ Human Brain Mapping 25:140–154(2005) ᭜ Cortical Regions Involved in Eye Movements, Shifts of Attention, and Gaze Perception Marie-He´le`ne Grosbras,1* Angela R. Laird,2 and Toma´s Paus1,3 1Cognitive Neuroscience Unit, Montreal Neurological Institute, McGill University, Montreal, Quebec, Canada 2Research Imaging Center, University of Texas Health Science Center, San Antonio, Texas 3Brain and Body Center, University of Nottingham, Nottingham, United Kingdom ᭜ ᭜ Abstract: Human vision is an active process that involves shifting attention across the visual scene, with or without moving the eyes. Such shifts of attention can be generated at will (endogenously) or be triggered automatically, i.e., generated in response to exogenous stimuli including socially relevant cues such as someone else’s gaze. What are the common and distinct brain mechanisms involved in these processes? To address this question, we carried out a quantitative effect-location meta-analysis of 59 brain-imaging experiments whose results were published using standardized coordinates. For each condition of interest, namely voluntary and visually triggered eye movements, voluntary and visually triggered (covert) shifts of attention, and perception of someone else’s gaze, we computed activation likelihood estimation (ALE) maps. Those maps represent at each voxel of the brain the probability of reporting a signal change related to the condition of interest. For eye movements, this analysis confirmed the spatial location of the frontal eye fields, supplementary eye fields, and parietal saccade-related regions. The map of covert shifts of attention demonstrated highest similarity with the map of saccadic eye movements. Gaze perception showed common activation likelihood with the other conditions in the right intraparietal sulcus and in the lateral precentral gyrus. -
Core-Example1.Pdf
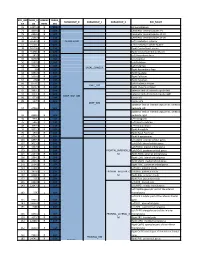
ROI_IND NUM_V HEMISP TISSUE_ SUBGROUP_0 SUBGROUP_1 SUBGROUP_2 ROI_NAME EX OX HERE SEG 95 12871.8 B WM corpus callosum 71 4899.8 B GM Cerebellar Vermal Lobules I-V 73 2858.8 B GM Cerebellar Vermal Lobules VIII-X 72 2266.9 B GM Cerebellar Vermal Lobules VI-VII 39 54582.6 L GM CEREBELLUM Left Cerebellum Exterior 41 15500.7 L WM Left Cerebellum White Matter 38 54379.4 R GM Right Cerebellum Exterior 40 15458.7 R WM Right Cerebellum White Matter 30 585.9 L GM Left Accumbens Area 37 3578.9 L GM Left Caudate 56 1597.6 L GM Left Pallidum 58 4942.3 L GM Left Putamen BASAL_GANGLIA 23 526 R GM Right Accumbens Area 36 3651.5 R GM Right Caudate 55 1638.8 R GM Right Pallidum 57 4726 R GM Right Putamen 60 8574.1 L GM Left Thalamus Proper DEEP_GM 59 8256.3 R GM Right Thalamus Proper 92 2887.7 L WM anterior limb of internal capsule left 91 3393.3 R WM anterior limb of internal capsule right DEEP_WM_GM 90 673.6 L WM fornix left 89 517.5 R WM fornix right DEEP_WM posterior limb of internal capsule inc. cerebral 94 2416.3 L WM peduncle left posterior limb of internal capsule inc. cerebral 93 2480.5 R WM peduncle right 32 993.7 L GM Left Amygdala 75 586.5 L GM Left Basal Forebrain 48 3597.7 L GM Left Hippocampus 31 1021.3 R GM Right Amygdala 76 593.1 R GM Right Basal Forebrain 47 3704.7 R GM Right Hippocampus 105 1897.7 L GM Left AOrG anterior orbital gyrus 137 3015.9 L GM Left LOrG lateral orbital gyrus 147 4637.3 L GM Left MOrG medial orbital gyrus 179 2915.7 L GM FRONTAL_INFERIOR_G Left POrG posterior orbital gyrus 104 2244.9 R GM M Right AOrG anterior orbital -

Subtemporal Transparahippocampal Amygdalohippocampectomy for Surgical Treatment of Mesial Temporal Lobe Epilepsy Technical Note
Subtemporal transparahippocampal amygdalohippocampectomy for surgical treatment of mesial temporal lobe epilepsy Technical note T. S. Park, M.D., Blaise F. D. Bourgeois, M.D., Daniel L. Silbergeld, M.D., and W. Edwin Dodson, M.D. Department of Neurology and Neurological Surgery, Washington University School of Medicine, and St. Louis Children's Hospital, St. Louis, Missouri Amygdalohippocampectomy (AH) is an accepted surgical option for treatment of medically refractory mesial temporal lobe epilepsy. Operative approaches to the amygdala and hippocampus that previously have been reported include: the sylvian fissure, the superior temporal sulcus, the middle temporal gyrus, and the fusiform gyrus. Regardless of the approach, AH permits not only extirpation of an epileptogenic focus in the amygdala and anterior hippocampus, but interruption of pathways of seizure spread via the entorhinal cortex and the parahippocampal gyrus. The authors report a modification of a surgical technique for AH via the parahippocampal gyrus, in which excision is limited to the anterior hippocampus, amygdala and parahippocampal gyrus while preserving the fusiform gyrus and the rest of the temporal lobe. Because transparahippocampal AH avoids injury to the fusiform gyrus and the lateral temporal lobe, it can be performed without intracarotid sodium amobarbital testing of language dominance and language mapping. Thus the operation would be particularly suitable for pediatric patients in whom intraoperative language mapping before resection is difficult. Key Words * amygdalohippocampectomy * complex partial seizure * parahippocampal gyrus * subtemporal approach Currently several different variations of temporal lobe resections are used for medically intractable complex partial seizures.[4,6,8,18,21,30,34] Among these operations is amygdalohippocampectomy (AH), first described in 1958 by Niemeyer,[16] who approached the amygdala and hippocampus through an incision on the middle temporal gyrus. -

Central Effects of Occipital Nerve Electrical Stimulation Studied by Functional Magnetic Resonance Imaging
Neuromodulation: Technology at the Neural Interface Received: October 28, 2009 First revision: July 7, 2010 Accepted: July 7, 2010 (onlinelibrary.wiley.com) DOI: 10.1111/j.1525-1403.2010.00312.x Central Effects of Occipital Nerve Electrical Stimulation Studied by Functional Magnetic Resonance Imagingner_312 46..57 Silvia Kovacs, MSc*, Ronald Peeters, PhD*, Dirk De Ridder, MD, PhD†, Mark Plazier, MD†, Tomas Menovsky, MD†, Stefan Sunaert, MD, PhD* Objective: To study the central effects of occipital nerve stimulation (ONS) using functional magnetic resonance imaging (fMRI). Materials and Methods: After phantom measurements, blocked design fMRI scanning was performed during intermittent ONS in a healthy volunteer with implanted electrodes connected to an external generator. To assess the effect of frequency and stimulation mode, seven different frequencies in either tonic or burst mode were generated by a neurostimulator. Results: A qualitative analysis of the main effect of ONS demonstrated significantly decreased activity within the bilateral primary visual, auditory, and somatosensory cortices and in the amygdala. Significant increased activity was observed bilaterally in the thalamus, frontal, and parietal areas and the cerebellum. Subsequently, quantitative analysis revealed that, unlike tonic mode stimulation, burst mode stimulation appeared to be frequency-dependent. Conclusions: This study demonstrates the feasibility and safety of fMRI studies with simultaneous ONS in a subject with exter- nalized electrodes. The activation and deactivation pattern induced by ONS depends on stimulation mode and frequency. Keywords: chronic pain, implantation, magnetic resonance imaging, occipital nerve stimulation, safety Conflict of Interest: Dr. DeRidder received an educational grant from St. Jude Medical. The other authors reported no conflicts of interest.