Flavonoids Derivatives from Arundina Graminifolia and Their Cytotoxicity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

National List of Vascular Plant Species That Occur in Wetlands 1996
National List of Vascular Plant Species that Occur in Wetlands: 1996 National Summary Indicator by Region and Subregion Scientific Name/ North North Central South Inter- National Subregion Northeast Southeast Central Plains Plains Plains Southwest mountain Northwest California Alaska Caribbean Hawaii Indicator Range Abies amabilis (Dougl. ex Loud.) Dougl. ex Forbes FACU FACU UPL UPL,FACU Abies balsamea (L.) P. Mill. FAC FACW FAC,FACW Abies concolor (Gord. & Glend.) Lindl. ex Hildebr. NI NI NI NI NI UPL UPL Abies fraseri (Pursh) Poir. FACU FACU FACU Abies grandis (Dougl. ex D. Don) Lindl. FACU-* NI FACU-* Abies lasiocarpa (Hook.) Nutt. NI NI FACU+ FACU- FACU FAC UPL UPL,FAC Abies magnifica A. Murr. NI UPL NI FACU UPL,FACU Abildgaardia ovata (Burm. f.) Kral FACW+ FAC+ FAC+,FACW+ Abutilon theophrasti Medik. UPL FACU- FACU- UPL UPL UPL UPL UPL NI NI UPL,FACU- Acacia choriophylla Benth. FAC* FAC* Acacia farnesiana (L.) Willd. FACU NI NI* NI NI FACU Acacia greggii Gray UPL UPL FACU FACU UPL,FACU Acacia macracantha Humb. & Bonpl. ex Willd. NI FAC FAC Acacia minuta ssp. minuta (M.E. Jones) Beauchamp FACU FACU Acaena exigua Gray OBL OBL Acalypha bisetosa Bertol. ex Spreng. FACW FACW Acalypha virginica L. FACU- FACU- FAC- FACU- FACU- FACU* FACU-,FAC- Acalypha virginica var. rhomboidea (Raf.) Cooperrider FACU- FAC- FACU FACU- FACU- FACU* FACU-,FAC- Acanthocereus tetragonus (L.) Humm. FAC* NI NI FAC* Acanthomintha ilicifolia (Gray) Gray FAC* FAC* Acanthus ebracteatus Vahl OBL OBL Acer circinatum Pursh FAC- FAC NI FAC-,FAC Acer glabrum Torr. FAC FAC FAC FACU FACU* FAC FACU FACU*,FAC Acer grandidentatum Nutt. -

The Orchid Flora of the Colombian Department of Valle Del Cauca Revista Mexicana De Biodiversidad, Vol
Revista Mexicana de Biodiversidad ISSN: 1870-3453 [email protected] Universidad Nacional Autónoma de México México Kolanowska, Marta The orchid flora of the Colombian Department of Valle del Cauca Revista Mexicana de Biodiversidad, vol. 85, núm. 2, 2014, pp. 445-462 Universidad Nacional Autónoma de México Distrito Federal, México Available in: http://www.redalyc.org/articulo.oa?id=42531364003 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Revista Mexicana de Biodiversidad 85: 445-462, 2014 Revista Mexicana de Biodiversidad 85: 445-462, 2014 DOI: 10.7550/rmb.32511 DOI: 10.7550/rmb.32511445 The orchid flora of the Colombian Department of Valle del Cauca La orquideoflora del departamento colombiano de Valle del Cauca Marta Kolanowska Department of Plant Taxonomy and Nature Conservation, University of Gdańsk. Wita Stwosza 59, 80-308 Gdańsk, Poland. [email protected] Abstract. The floristic, geographical and ecological analysis of the orchid flora of the department of Valle del Cauca are presented. The study area is located in the southwestern Colombia and it covers about 22 140 km2 of land across 4 physiographic units. All analysis are based on the fieldwork and on the revision of the herbarium material. A list of 572 orchid species occurring in the department of Valle del Cauca is presented. Two species, Arundina graminifolia and Vanilla planifolia, are non-native elements of the studied orchid flora. The greatest species diversity is observed in the montane regions of the study area, especially in wet montane forest. -

Cytological Studies on Arundina Graminifolia (Orchidaceae)
_??_1987 by Cytologia, TokyoCytologia 52: 267 -273 , 1987 Cytological Studies on Arundina graminifolia (Orchidaceae) Y. H. Lee Botany Department, National University of Singapore, Singapore Accepted March 17, 1986 Arundiana graminifolia (Don.) Hochr. is a terrestrial orchid whose native habitat extends from Sri Lanka and India through South East Asia to Tahiti (excluding the Philippines) . As there are considerable morphological variations among different populations , some taxonomists classified the genus into as many as 8 species (Sheehan and Sheehan 1983) . However, Holttum (1964) considered it as one highly variable species and presented good supporting arguments. His viewpoint is generally accepted at present. There were very few reports on cytological studies on this species. Pancho (1965) reported a somatic chromosome number of 32 for his material, while Tanaka (1965) as well as Sharma and Chatterji (1966) reported 40 chromosomes. The latter also noted some meiotic irregul arities as early separation of homologous chromosomes and laggards at anaphase I. More recently, Mehra and Vij (1970) observed a haploid set of 20 chromosomes in gametes of this species. Further observations on meiotic chromosome behaviour are described in this paper. Materials and methods Arundina graminifolia is commonly grown in Singapore and Malaysia. Each plant often consists of many stems growing close together. One such plant with a height of about 1 meter growing in the garden of the Botany Department of the National University of Singapore was used for the present study. Young flower buds at desirable stages were collected in the morning. These pollinia were dissected out and fixed in 45% acetic acid for 10 minutes at room temper ature. -

Tropicalexotique First Q 2020
Plant List TropicalExotique First Q 2020 Your Size when shipped When mature, well grown size CAD/Plant Total (CAD) Name Order P1 Aerangis fastuosa single growth, blooming size small plant 35 - P2 Aerides multiflorum single growth, blooming size medium plant 30 - P3 Aerides odorata "Pink form" single growth, blooming size medium plant 25 - P4 Aerides rosea single growth, blooming size medium plant 30 - P5 Amesiella minor single growth, blooming size miniature 50 - P6 Amesiella monticola single growth, blooming size small plant 30 - P7 Angraecum didieri seedling size medium plant 25 - P8 Anthogonium gracile per bulb small plant 25 - P9 Appendicula elegans 3-5 bulb plant small plant 30 - P10 Arachnis labrosa single growth, blooming size large plant 40 - P11 Armodorum siamemse blooming size medium plant 25 - P12 Arundina graminifolia (mini type, dark red) Single growth small plant 40 - P13 Arundina graminifolia (mini type, pink) multi-growth, blooming size medium plant 40 - P14 Ascocentrum (Holcoglossum) himalaicum single growth, blooming size medium plant 60 - P15 Ascocentrum (Vanda) ampullaceum single growth medium plant 30 - P16 Ascocentrum (Vanda) ampullaceum forma alba seedling size medium plant 25 - P17 Ascocentrum (Vanda) ampullaceum forma aurantiacum single growth medium plant 45 - P18 Ascocentrum (Vanda) christensonianum single growth, blooming size medium plant 40 - P19 Ascocentrum (Vanda) curvifolium single growth medium plant 20 - P20 Ascocentrum (Vanda) curvifolium "Pink form" single growth medium plant 30 - P21 Ascocentrum (Vanda) -

North East Ecoregion Report
NORTH-EAST ECOREGION BIODIVERSITY STRATEGY AND ACTION PLAN (A part of National Biodiversity Strategy and Action Plan process) R.S. TRIPATHI* AND S.K. BARIK** Department of Botany North-Eastern Hill University SHILLONG – 793 022 (*Coordinator, North-East Ecoregion Working Group; ** Member, North-East Ecoregion Working Group) Submitted to: Ministry of Environment and Forests Government of India New Delhi Acknowledgements The authors acknowledge with thanks the valuable inputs received from the North- Eastern Ecoregional Working Group members at different stages of preparation of this report. The information provided by the local knowledgeable persons including the village/elaka chiefs and other community/village level functionaries were quite useful and the authors are grateful to them for their excellent cooperation and suggestions. The critical comments from the experts enhanced the quality of the report and the write-ups on thematic topics from the theme experts helped a great deal in finalizing the report. The names of these experts and local knowledgeable persons are listed in Annexures and the authors are thankful to each one of them. The comments of Prof. P.C. Bhattacharjee, Dr. A. Chadhury, Shri Ashish Kothari and other workers from Kalpavriksa were particularly of great help. The report has freely drawn information from the relevant State and Sub-state reports, for which the authors are grateful to all the coordinators, members and others who were involved in preparation of those documents. Besides, the State Forestry Action Plans of all the 8 north-eastern states were consulted and used during the preparation of this report. The authors are thankful to all the PCCFs and the authors of SFAPs for the same. -

National Wetland Plant List: 2016 Wetland Ratings
Lichvar, R.W., D.L. Banks, W.N. Kirchner, and N.C. Melvin. 2016. The National Wetland Plant List: 2016 wetland ratings. Phytoneuron 2016-30: 1–17. Published 28 April 2016. ISSN 2153 733X THE NATIONAL WETLAND PLANT LIST: 2016 WETLAND RATINGS ROBERT W. LICHVAR U.S. Army Engineer Research and Development Center Cold Regions Research and Engineering Laboratory 72 Lyme Road Hanover, New Hampshire 03755-1290 DARIN L. BANKS U.S. Environmental Protection Agency, Region 7 Watershed Support, Wetland and Stream Protection Section 11201 Renner Boulevard Lenexa, Kansas 66219 WILLIAM N. KIRCHNER U.S. Fish and Wildlife Service, Region 1 911 NE 11 th Avenue Portland, Oregon 97232 NORMAN C. MELVIN USDA Natural Resources Conservation Service Central National Technology Support Center 501 W. Felix Street, Bldg. 23 Fort Worth, Texas 76115-3404 ABSTRACT The U.S. Army Corps of Engineers (Corps) administers the National Wetland Plant List (NWPL) for the United States (U.S.) and its territories. Responsibility for the NWPL was transferred to the Corps from the U.S. Fish and Wildlife Service (FWS) in 2006. From 2006 to 2012 the Corps led an interagency effort to update the list in conjunction with the U.S. Environmental Protection Agency (EPA), the FWS, and the USDA Natural Resources Conservation Service (NRCS), culminating in the publication of the 2012 NWPL. In 2013 and 2014 geographic ranges and nomenclature were updated. This paper presents the fourth update of the list under Corps administration. During the current update, the indicator status of 1689 species was reviewed. A total of 306 ratings of 186 species were changed during the update. -

107. ARUNDINA Blume, Bijdr. 401. 1825. 竹叶兰属 Zhu Ye Lan Shu Chen Xinqi (陈心启 Chen Sing-Chi); Stephan W
Flora of China 25: 314–315. 2009. 107. ARUNDINA Blume, Bijdr. 401. 1825. 竹叶兰属 zhu ye lan shu Chen Xinqi (陈心启 Chen Sing-chi); Stephan W. Gale Herbs, terrestrial. Rhizome ovoid, stout; roots many, filiform, slender, fibrous. Stem erect, tufted at base, reedlike, unbranched or occasionally branched when older, leafy. Leaves ± distichous, linear-lanceolate, conduplicate and sheathing at base, apex acute. Inflorescence terminal, unbranched or occasionally shortly branched, few to many flowered; floral bracts persistent, triangular, small. Flowers resupinate, opening one at a time, large; pedicel and ovary elongate, slender. Sepals similar, elliptic or lanceolate, apex acute; lateral sepals usually connivent below lip. Petals spreading, ovate-elliptic or obovate, apex acute; lip adnate to base of column, obovate, not spurred, 3-lobed; disk with 3 longitudinal pubescent lamellae; lateral lobes erect, embracing column, rounded; mid-lobe spreading, apex divided. Column long, straight, dilated and narrowly winged toward apex; anther terminal, incumbent; pollinia 8, in 2 groups, ± waxy, with short caudicles, attached to sticky viscidia; stigma transversely oblong, broad. Capsule cylindric-fusiform, large, ridged. One highly variable species: from Nepal, NE and S India, and Bhutan, through S China, to SE Asia and S Japan, introduced and naturalized in the Pacific islands and the Neotropics. 1. Arundina graminifolia (D. Don) Hochreutiner, Bull. New 40 × 7–9 mm. Petals ovate-elliptic, 25–40 × 13–15 mm; lip 25– York Bot. Gard. 6: 270. 1910. 40 × 12–24 mm, apical margin undulate; lateral lobes incurved, embracing column, rounded; mid-lobe subsquare, 8–16 × 10– 竹叶兰 zhu ye lan 16 mm, apex shallowly divided; disk with 3 (rarely 5) lamellae. -

Inventory of Vascular Plants of the Kahuku Addition, Hawai'i
CORE Metadata, citation and similar papers at core.ac.uk Provided by ScholarSpace at University of Hawai'i at Manoa PACIFIC COOPERATIVE STUDIES UNIT UNIVERSITY OF HAWAI`I AT MĀNOA David C. Duffy, Unit Leader Department of Botany 3190 Maile Way, St. John #408 Honolulu, Hawai’i 96822 Technical Report 157 INVENTORY OF VASCULAR PLANTS OF THE KAHUKU ADDITION, HAWAI`I VOLCANOES NATIONAL PARK June 2008 David M. Benitez1, Thomas Belfield1, Rhonda Loh2, Linda Pratt3 and Andrew D. Christie1 1 Pacific Cooperative Studies Unit (University of Hawai`i at Mānoa), Hawai`i Volcanoes National Park, Resources Management Division, PO Box 52, Hawai`i National Park, HI 96718 2 National Park Service, Hawai`i Volcanoes National Park, Resources Management Division, PO Box 52, Hawai`i National Park, HI 96718 3 U.S. Geological Survey, Pacific Island Ecosystems Research Center, PO Box 44, Hawai`i National Park, HI 96718 TABLE OF CONTENTS ABSTRACT.......................................................................................................................1 INTRODUCTION...............................................................................................................1 THE SURVEY AREA ........................................................................................................2 Recent History- Ranching and Resource Extraction .....................................................3 Recent History- Introduced Ungulates...........................................................................4 Climate ..........................................................................................................................4 -

Evaluation of Indonesian Mangrove Xylocarpus Granatum Leaves Ethyl
www.nature.com/scientificreports OPEN Evaluation of Indonesian mangrove Xylocarpus granatum leaves ethyl acetate extract as potential anticancer drug Jason Darmadi1, Razethy Rahayu Batubara1, Sandiego Himawan1, Norma Nur Azizah2, Hilyatushalihah Kholis Audah1, Ade Arsianti2,3, Evi Kurniawaty4, Intan Safnar Ismail5, Irmanida Batubara6,7 & Kholis Abdurachim Audah1,8* Local Xylocarpus granatum leaves were extracted by ethyl acetate solvent and characterized by TLC fngerprinting and 2D 1H NMR spectroscopy to contain phenolic compounds as well as several organic and amino acids as metabolic byproducts, such as succinic acid and acetic acid. Traces of favonoids and other non-categorized phenolic compounds exhibited intermediate antioxidant activity (antioxidant IC50 84.93 ppm) as well as anticancer activity against HeLa, T47D, and HT-29 cell lines; which the latter being most efective against HT-29 with Fraction 5 contained the strongest activity (anticancer IC50 23.12 ppm). Extracts also behaved as a natural growth factor and nonlethal towards brine shrimps as well as human adipose-derived stem cell hADSC due to antioxidative properties. A stability test was performed to examine how storage conditions factored in bioactivity and phytochemical structure. Extracts were compared with several studies about X. granatum leaves extracts to evaluate how ethnogeography and ecosystem factored on biologically active compounds. Further research on anticancer or antioxidant mechanism on cancer cells is needed to determine whether the extract is suitable as a candidate for an anticancer drug. Indonesia is regarded as one of the richest countries in terms of biodiversity, housing approximately 11% of the world’s fora and fauna 1. Despite the large quantities of diferent vascular plants reported in Indonesia, as well as their cultural signifcance for traditional herbal medicines or bioprospecting program is not yet working properly for Indonesia’s pharmaceutical industry, with 95% of pharmaceutics are imported products 2. -
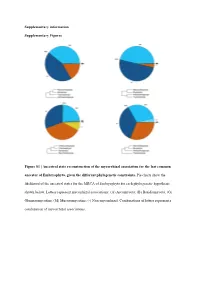
Ancestral State Reconstruction of the Mycorrhizal Association for the Last Common Ancestor of Embryophyta, Given the Different Phylogenetic Constraints
Supplementary information Supplementary Figures Figure S1 | Ancestral state reconstruction of the mycorrhizal association for the last common ancestor of Embryophyta, given the different phylogenetic constraints. Pie charts show the likelihood of the ancestral states for the MRCA of Embryophyta for each phylogenetic hypothesis shown below. Letters represent mycorrhizal associations: (A) Ascomycota; (B) Basidiomycota; (G) Glomeromycotina; (M) Mucoromycotina; (-) Non-mycorrhizal. Combinations of letters represent a combination of mycorrhizal associations. Austrocedrus chilensis Chamaecyparis obtusa Sequoiadendron giganteum Prumnopitys taxifolia Prumnopitys Prumnopitys montana Prumnopitys Prumnopitys ferruginea Prumnopitys Araucaria angustifolia Araucaria Dacrycarpus dacrydioides Dacrycarpus Taxus baccata Podocarpus oleifolius Podocarpus Afrocarpus falcatus Afrocarpus Ephedra fragilis Nymphaea alba Nymphaea Gnetum gnemon Abies alba Abies balsamea Austrobaileya scandens Austrobaileya Abies nordmanniana Thalictrum minus Thalictrum Abies homolepis Caltha palustris Caltha Abies magnifica ia repens Ranunculus Abies religiosa Ranunculus montanus Ranunculus Clematis vitalba Clematis Keteleeria davidiana Anemone patens Anemone Tsuga canadensis Vitis vinifera Vitis Tsuga mertensiana Saxifraga oppositifolia Saxifraga Larix decidua Hypericum maculatum Hypericum Larix gmelinii Phyllanthus calycinus Phyllanthus Larix kaempferi Hieronyma oblonga Hieronyma Pseudotsuga menziesii Salix reinii Salix Picea abies Salix polaris Salix Picea crassifolia Salix herbacea -

Orchids Diversity in the Sicikeh-Cikeh Forest, North Sumatra, Indonesia
BIODIVERSITAS ISSN: 1412-033X Volume 20, Number 4, April 2019 E-ISSN: 2085-4722 Pages: 1087-1096 DOI: 10.13057/biodiv/d200421 Orchids Diversity in the Sicikeh-Cikeh Forest, North Sumatra, Indonesia SRI HARTINI Research Centre for Plant Conservation and Botanic Gardens (Bogor Botanic Gardens), Indonesian Institute of Sciences. Jl. Ir. H. Juanda No. 13, Bogor 16122, West Java, Indonesia. Tel./fax.: +62-251-8322-187, ♥email: [email protected] Manuscript received: 19 February 2019. Revision accepted: 23 March 2019. Abstract. Hartini S. 2019. Orchids Diversity in the Sicikeh-Cikeh Forest, North Sumatra, Indonesia. Biodiversitas 20: 1087-1096. Sicikeh-cikeh forest includes three forest areas, namely Adian Tinjoan Customary Forest, Adian Tinjoan Protection Forest, and Taman Wisata Alam Sicikeh-cikeh. Typical vegetation of this area is mountain forest with large diversity of plant species. Among the species, orchid is one of the potential plants found diversely in this location and has not yet been recorded. The exploration activities were conducted at Sicikeh-cikeh forest. Orchid exploration was conducted to collect living plants for ex situ conservation purpose. Orchid inventory, to record orchid diversity in this area, was based on plant collection by purposive random sampling. The results of the study recorded approximately 102 different species from 30 genera of orchids in this area. Typical epiphytic and terrestrial highland orchids were found and very common in Sumatra. Among the species found are endemic Sumatra, such as Coelogyne brachygyne, Coelogyne salmonicolor, Dendrobium kruiense, Epigeneium pulchellum, Thrixspermum gombakense, Corybas stenotribonos and Paphiopedilum tonsum. Interesting terrestrial orchids include Kuhlhasseltia javanica, Neuwiedia zollingeri var. javanica, Paphiopedilum tonsum, Phaius callosus, Corybas stenotribonos, Calanthe aurantiaca, Calanthe chrysoglossoides, and Calanthe pulchra.