Evaluation of Dehydration Mechanism During Heating of Hydrous Asteroids Based on Mineralogical and Chemical Analysis of Naturally and Experimentally Heated CM Chondrites
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Finegrained Precursors Dominate the Micrometeorite Flux
Meteoritics & Planetary Science 47, Nr 4, 550–564 (2012) doi: 10.1111/j.1945-5100.2011.01292.x Fine-grained precursors dominate the micrometeorite flux Susan TAYLOR1*, Graciela MATRAJT2, and Yunbin GUAN3 1Cold Regions Research and Engineering Laboratory, 72 Lyme Road, Hanover, New Hampshire 03755–1290, USA 2University of Washington, Seattle, Washington 98105, USA 3Geological & Planetary Sciences MC 170-25, Caltech, Pasadena, California 91125, USA *Corresponding author. E-mail: [email protected] (Received 15 May 2011; revision accepted 22 September 2011) Abstract–We optically classified 5682 micrometeorites (MMs) from the 2000 South Pole collection into textural classes, imaged 2458 of these MMs with a scanning electron microscope, and made 200 elemental and eight isotopic measurements on those with unusual textures or relict phases. As textures provide information on both degree of heating and composition of MMs, we developed textural sequences that illustrate how fine-grained, coarse-grained, and single mineral MMs change with increased heating. We used this information to determine the percentage of matrix dominated to mineral dominated precursor materials (precursors) that produced the MMs. We find that at least 75% of the MMs in the collection derived from fine-grained precursors with compositions similar to CI and CM meteorites and consistent with dynamical models that indicate 85% of the mass influx of small particles to Earth comes from Jupiter family comets. A lower limit for ordinary chondrites is estimated at 2–8% based on MMs that contain Na-bearing plagioclase relicts. Less than 1% of the MMs have achondritic compositions, CAI components, or recognizable chondrules. Single mineral MMs often have magnetite zones around their peripheries. -

Ancient Martian Life in Allan Hills 84001? Status of Some Current Controversies
Workshop on the Issue of Martian Meteorites 7043.pdf ANCIENT MARTIAN LIFE IN ALLAN HILLS 84001? STATUS OF SOME CURRENT CONTROVERSIES. A. H. Treiman, Lunar and Planetary Institute, 3600 Bay Area Boulevard, Houston TX 77058, USA ([email protected]). Four lines of evidence were taken to suggest that ALH Also, it is possible that the elongate magnetites formed at 84001 contained traces of ancient martian life preserved in high T, but after the carbonates formed [5]. Magnetites are its carbonate mineral masses [1]: abundance of organic also present in void spaces in the carbonate, either as the compounds (PAHs), disequilibrium mineral assemblages, product of decarbonation at high T [21] or as entrapped from morphology of sub-micron magnetite crystals, and presence the aqueous fluid that deposited the carbonates [22]. of objects comparable in size and shape to bacteria. This Summary. There is no consensus on the formation T or evidence is predicated on the carbonate globules having mechanism of carbonate masses. The bulk of evidence, in formed at temperatures conducive to life. Here, I review my opinion, is not consistent with T>400°C [4,6,7]. At pres- evidence on carbonate formation temperature, martian ori- ent, there seems no way to tell whether the carbonates gin of organic compounds, and bacteria-shaped objects. formed at moderate or low T: hotter or colder than ~150°C. Formation Temperature: A precondition for signs of Organic Matter: McKay et al. [1] presented evidence life associated with the carbonate masses [1] is that the that the carbonate pancakes in ALH 84001 are enriched in masses formed at T amenable to life (as we know it) [2], polycyclic aromatic hydrocarbons, PAHs, which are organic <~150°C. -

Chapter 2 TEMPERATURES of AQUEOUS ALTERATION ON
46 Chapter 2 TEMPERATURES OF AQUEOUS ALTERATION ON CARBONACEOUS CHONDRITE PARENT BODIES Weifu Guo 1, Michael Zolensky 2 and John M. Eiler 1 1. Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, CA, 91125, USA 2. NASA Johnson Space Center, Houston, TX, USA 47 ABSTRACT Application of stepped phosphoric acid digestion and carbonate clumped isotope thermometry to carbonates in carbonaceous chondrites ⎯ GRO 95577 (CR1), Orgueil (CI) and Tagish Lake (ungrouped C2) ⎯ indicates that aqueous alterations on carbonaceous chondrite parent bodies occurred from -31 to 65˚C from fluids with 18 17 δ OVSMOW values of -29.7‰ to 11.8‰ and δ OVSMOW of -14.9‰ to 7.6‰. The estimated carbonate formation temperatures decrease in the order: calcite > dolomite > breunnerite. Based on independent constraints on the ages of these carbonates and models of the evolution of the oxygen isotope compositions of parent body waters, this trend indicates that the parent bodies were cooling as the aqueous alteration proceeded. We estimate that aqueous alteration on the carbonaceous chondrite parent bodies started within 1-2 million years after the accretion of those parent bodies, and that the alteration temperatures decreased from 34˚C to 18˚C in the first ~4 million years and further to - 20˚C after a total of ~6.5 million years. Our results provide the first direct measurements of the low-temperature cooling histories of C1 and C2 carbonaceous chondrite parent bodies. 1. INTRODUCTION Aqueous alteration of primitive meteorites is among the earliest and most widespread geological processes in the solar system, occurring within the first tens of million of years of solar system history (Brearley, 2006). -

(2000) Forging Asteroid-Meteorite Relationships Through Reflectance
Forging Asteroid-Meteorite Relationships through Reflectance Spectroscopy by Thomas H. Burbine Jr. B.S. Physics Rensselaer Polytechnic Institute, 1988 M.S. Geology and Planetary Science University of Pittsburgh, 1991 SUBMITTED TO THE DEPARTMENT OF EARTH, ATMOSPHERIC, AND PLANETARY SCIENCES IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN PLANETARY SCIENCES AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY FEBRUARY 2000 © 2000 Massachusetts Institute of Technology. All rights reserved. Signature of Author: Department of Earth, Atmospheric, and Planetary Sciences December 30, 1999 Certified by: Richard P. Binzel Professor of Earth, Atmospheric, and Planetary Sciences Thesis Supervisor Accepted by: Ronald G. Prinn MASSACHUSES INSTMUTE Professor of Earth, Atmospheric, and Planetary Sciences Department Head JA N 0 1 2000 ARCHIVES LIBRARIES I 3 Forging Asteroid-Meteorite Relationships through Reflectance Spectroscopy by Thomas H. Burbine Jr. Submitted to the Department of Earth, Atmospheric, and Planetary Sciences on December 30, 1999 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Planetary Sciences ABSTRACT Near-infrared spectra (-0.90 to ~1.65 microns) were obtained for 196 main-belt and near-Earth asteroids to determine plausible meteorite parent bodies. These spectra, when coupled with previously obtained visible data, allow for a better determination of asteroid mineralogies. Over half of the observed objects have estimated diameters less than 20 k-m. Many important results were obtained concerning the compositional structure of the asteroid belt. A number of small objects near asteroid 4 Vesta were found to have near-infrared spectra similar to the eucrite and howardite meteorites, which are believed to be derived from Vesta. -
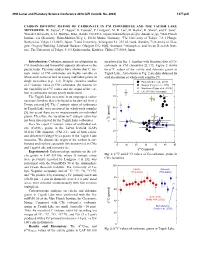
CARBON ISOTOPIC RATIOS of CARBONATE in CM CHONDRITES and the TAGISH LAKE METEORITE. W. Fujiya1, P. Hoppe2, K. Fukuda3, P. Lindgren4, M
49th Lunar and Planetary Science Conference 2018 (LPI Contrib. No. 2083) 1377.pdf CARBON ISOTOPIC RATIOS OF CARBONATE IN CM CHONDRITES AND THE TAGISH LAKE METEORITE. W. Fujiya1, P. Hoppe2, K. Fukuda3, P. Lindgren4, M. R. Lee5, M. Koike6, K. Shirai6, and Y. Sano6. 1Ibaraki University, 2-1-1 Bunkyo, Mito, Ibaraki 310-8512, Japan ([email protected]), 2Max Planck Institute for Chemistry, Hahn-Meitner-Weg 1, 55128 Mainz, Germany, 3The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan, 4Lund University, Sölvegatan 12, 223 62 Lund, Sweden, 5University of Glas- gow, Gregory Building, Lilybank Gardens, Glasgow G12 8QQ, Scotland, 6Atmosphere and Ocean Research Insti- tute, The University of Tokyo, 5-1-5 Kashiwanoha, Kashiwa, Chiba 277-8564, Japan. Introduction: Carbonate minerals are ubiquitous in are plotted in Fig. 1, together with literature data of Ca- CM chondrites and formed by aqueous alteration in the carbonate in CM chondrites [2,3,7]. Figure 2 shows parent body. Previous studies have shown that C iso- the13C values of the calcite and dolomite grains in topic ratios of CM carbonates are highly variable at Tagish Lake. Also shown in Fig. 2 are data obtained by whole-rock scales as well as among individual grains in acid dissolution of whole-rock samples [5]. single meteorites [e.g., 1-3]. Despite extensive studies Paris (Vacher et al., 2017) on C isotopic ratios of CM carbonates, the reasons for 100 Nogoya (Fujiya et al., 2016) the variability in 13C values and the origin of the car- Murchison (Fujiya et al., 2015) bon in carbonates remain poorly understood. -

The Sutter's Mill CM Chondrite and the Tissint Shergottite
44th Lunar and Planetary Science Conference (2013) 2376.pdf THE SUTTER’S MILL CM CHONDRITE AND THE TISSINT SHERGOTTITE: FIRST DATA FROM THE NASA AMES THERMOLUMINESCENCE LABORATORY. Derek W. G. Sears, Space Science and Astrobiology Division, MS245-3, NASA Ames Research Center, Moffett Field, Mountain View, CA 94035. ([email protected]). Introduction: Studies of the natural TL of meteor- The two fragments gave almost identical results, the ites provides information on their recent thermal and only difference being the presence of high- temperature radiation history and have application in the study of induced TL in the glass-rich fragment. The TL sensitiv- terrestrial age, orbit, and – in the case of Antarctic me- ities and peak temperatures for the two chips were teorites – concentration mechanisms. On the other identical within experimental limits, but the high tem- hand, studies of the induced TL provide unique in- perature component precluded measuring peak width in sights into metamorphic history of meteorites. TL sen- the glass-rich sample. The data are plotted in Fig. 1, sitivity (induced TL normalized to the induced TL of along with meteorites for which data currently exist the Dhajala H3.8 chondrite) has a dynamic range and [5]. The TL sensitivitiy, TL peak temperature and TL precision not matched by any other technique [1]. Dur- peah width of Tissint are very similar to those of Sher- ing the installation of a TL laboratory at NASA Ames gotty. The TL sensitivity of Tissint, while low in com- Research Center two meteorites with considerable parison to most meteorites, is at the high end of the community interest became available for research and range observed for shergottites. -

Systematics and Evaluation of Meteorite Classification 19
Weisberg et al.: Systematics and Evaluation of Meteorite Classification 19 Systematics and Evaluation of Meteorite Classification Michael K. Weisberg Kingsborough Community College of the City University of New York and American Museum of Natural History Timothy J. McCoy Smithsonian Institution Alexander N. Krot University of Hawai‘i at Manoa Classification of meteorites is largely based on their mineralogical and petrographic charac- teristics and their whole-rock chemical and O-isotopic compositions. According to the currently used classification scheme, meteorites are divided into chondrites, primitive achondrites, and achondrites. There are 15 chondrite groups, including 8 carbonaceous (CI, CM, CO, CV, CK, CR, CH, CB), 3 ordinary (H, L, LL), 2 enstatite (EH, EL), and R and K chondrites. Several chondrites cannot be assigned to the existing groups and may represent the first members of new groups. Some groups are subdivided into subgroups, which may have resulted from aster- oidal processing of a single group of meteorites. Each chondrite group is considered to have sampled a separate parent body. Some chondrite groups and ungrouped chondrites show chemi- cal and mineralogical similarities and are grouped together into clans. The significance of this higher order of classification remains poorly understood. The primitive achondrites include ureil- ites, acapulcoites, lodranites, winonaites, and silicate inclusions in IAB and IIICD irons and probably represent recrystallization or residues from a low-degree partial melting of chondritic materials. The genetic relationship between primitive achondrites and the existing groups of chondritic meteorites remains controversial. Achondrites resulted from a high degree of melt- ing of chondrites and include asteroidal (angrites, aubrites, howardites-diogenites-eucrites, mesosiderites, 3 groups of pallasites, 15 groups of irons plus many ungrouped irons) and plane- tary (martian, lunar) meteorites. -

Revision 1 Mineralogy of the 2019 Aguas Zarcas (CM2) Carbonaceous
This is the peer-reviewed, final accepted version for American Mineralogist, published by the Mineralogical Society of America. The published version is subject to change. Cite as Authors (Year) Title. American Mineralogist, in press. DOI: https://doi.org/10.2138/am-2021-7815. http://www.minsocam.org/ 1 Revision 1 2 3 Mineralogy of the 2019 Aguas Zarcas (CM2) carbonaceous chondrite 4 meteorite fall 5 6 Laurence A.J. Garvie 7 Center for Meteorite Studies, Arizona State University, Tempe, Arizona 85287–6004, 8 USA 9 10 ABSTRACT 11 The 2019 Aguas Zarcas CM2 meteorite is the most significant carbonaceous chondrite 12 CM2 fall since Murchison in 1969. Samples collected immediately following the fall, and 13 studied here, provide the rare opportunity to analyze the bulk mineralogy of a CM2 largely 14 free of terrestrial contamination. Bulk samples were analyzed by powder X-ray diffraction 15 (XRD), thermal gravimetric (TG) analysis, evolved gas analysis (EGA), and scanning 16 electron microscopy (SEM) with an electron-probe micro-analyzer (EPMA). Water- 17 extracted salts were analyzed by XRD. In hand specimen, the stones are brecciated and 18 dominated by chondrule-rich and –poor lithologies, and locally, a matrix-rich lithology. 19 Powder XRD patterns from multiple stones are dominated by reflections from serpentine 20 group minerals, on which are superimposed reflections for ferrotochilinite, 1:1 regularly 21 interstratified ferrotochilinite/cronstedtite, anhydrous silicates, calcite, pentlandite, 22 pyrrhotite, and minor phases. Reflections for magnetite are present only from a metal-rich 23 breccia clast. The serpentine XRD reflections from the chondrule-rich and –poor 24 lithologies match those from 1T cronstedtite, whereas those from the matrix-rich lithology 25 match the 1M polytype. -

Chondritic Meteorites and the High-Temperature Nebular Origins of Their Components
Chondrites and the Protoplanetary Disk ASP Conference Series, Vol. 341, 2005 A. N. Krot, E. R. D. Scott, & B. Reipurth, eds. Chondritic Meteorites and the High-Temperature Nebular Origins of Their Components Edward R. D. Scott and Alexander N. Krot Hawaii Institute of Geophysics and Planetology, University of Hawaii at Manoa, Honolulu, Hawaii 96822, USA Abstract. We present an overview of the chondrite groups focusing on the major con- straints that can be derived on thermal history of silicates in the solar nebula from the min- eralogical, chemical, and isotopic properties of the components in the least-altered chon- drites. Recent advances in developing a chronology for the formation of CAIs and chon- drules and plausible models for their oxygen isotopic compositions provide the basis for understanding their origin. Evidence from short-lived and long-lived isotopes, oxygen iso- topes, nuclear isotopic effects and petrologic studies all suggest that refractory inclusions and grains were the first solids to form in the protosolar disk, probably within a period of <0.3 Myr, when the protosun was accreting rapidly (possibly as a class 0 or I protostar). Refractory inclusions formed in an 16O-rich, reducing environment of near-solar composi- tion, <10-4 bar pressure, and temperatures >1300 K. Most chondrules appear to have formed 1-3 Myr after refractory inclusions, when the protosun was accreting more slowly. Chondrules in a single chondrite group probably formed over a much shorter period. Sev- eral types of chondrules formed under diverse conditions that were generally more oxidiz- ing with lower ambient temperatures and higher total pressures or high dust/gas ratios, so that liquids were stable for hours. -

Carbonaceous Chondrite Impact Melts
CosmoELEMENTS CARBONACEOUS CHONDRITE IMPACT MELTS Nicole G. Lunning1,2 and Catherine M. Corrigan2 The term “carbonaceous” in their name is a bit of a misnomer. Although 1811-5209/17/0013-0068$0.00 DOI: 10.2113/gselements.13.1.68 a few groups within this meteorite class are somewhat carbon-rich (e.g. CI and CM chondrites contain up to wt% quantities of elemental Collisions between planetary bodies (such as asteroids colliding with carbon), carbonaceous chondrites do not consistently contain more one another or with planets) have played a role in the geologic evolution carbon than other stony meteorite classes (Krot et al. 2003; Scott and of our Solar System since the formation of planetesimals, the earliest Krot 2003). kilometer-scale bodies. Shock damage from collisional impacts leaves evidence on surviving planetary materials that range in scale from MISSING IMPACT MELTS kilometer-sized craters to nanometer-sized mineral structural defects. Scott et al. (1992) called attention to the dearth of impact melted carbo- Impact shock-induced melting is thought to be a common consequence naceous chondrite material. The lack of carbonaceous chondrite impact of collisions throughout the Solar System. While ordinary chondrites, melts might be explained by the meteorites higher volatile concen- martian, and lunar meteorites all exhibit signs of having been melted by trations which may prevent the formation of cohesive melts. Many impacts, until very recently, no impact melts of primitive carbonaceous researchers have been searching for these melt features to little avail. chondrites had been recognized (Lunning et al. 2016a). CARBONACEOUS CHONDRITE IMPACT MELTS CARBONACEOUS CHONDRITES – FOUND! Carbonaceous chondrites include the most primitive known Solar Recently, in three separate meteorites, five objects have been identified System materials and hold important clues for understanding the origin that appear to be carbonaceous chondrite impact melts. -

Tagish Lake -- a Meteorite from the Far Reaches of the Asteroid Belt
PSRD: Tagish Lake Meteorite http://www.psrd.hawaii.edu/Dec02/TagishLake.html posted December 12, 2002 Tagish Lake -- A Meteorite from the Far Reaches of the Asteroid Belt --- A new type of primitive meteorite with much to tell us about the formation of the solar system. Written by David W. Mittlefehldt NASA, Johnson Space Center Pieces of a 56-metric-ton meteorite rained down over a wide area of Canada on January 18, 2000. Many pieces landed on the frozen Tagish Lake, allowing scientists to recover numerous samples, and giving the meteorite its name. Studies show that the meteorite is intermediate in composition between the two most primitive groups of chondrite meteorites, CI and CM carbonaceous chondrites. Observations of its trajectory allowed scientists to calculate its path through the solar system. The calculations show that it hails from the outer asteroid belt, in a place where dark, carbon- and water-rich asteroids reside. Tagish Lake is a new type of primitive meteorite that will surely shed light on how the solar system formed. Reference: Mittlefehldt, David W. (2002) Geochemistry of the ungrouped carbonaceous chondrite Tagish Lake, the anomalous CM chondrite Bells, and comparison with CI and CM chondrites, Meteoritics and Planetary Science, v. 37, p. 703-712. Arctic Fireball Every year, the Earth is pelted by debris that occupies the void between the planets. This debris is mostly dust- to sand-sized rocky bits shed by comets or asteroids. You, dear web-surfer, may have witnessed the recent spectacular Leonid meteor shower. This occurs when the Earth plows through dust expelled from comet Temple-Tuttle as it travels through the inner solar system -- in 2002 we went through a particularly concentrated dust tail, resulting in the excellent Leonid display. -

Meteorite Evidence for the Accretion and Collisional Evolution of Asteroids
Scott: Accretion and Collisional Evolution of Asteroids 697 Meteorite Evidence for the Accretion and Collisional Evolution of Asteroids Edward R. D. Scott University of Hawai‘i Meteorites contain a record of impacts during all stages of asteroid origin and evolution: the formation and accretion of chondritic particles; the alteration, metamorphism and melting of asteroids; and the erosion and disruption of asteroids by hypervelocity impacts. A review of meteorite classification shows that numerous meteorites are not readily classified because they do not fit simple models for asteroid formation and evolution that assume impacts were only important during the final stage of asteroid evolution and because of inadequate understanding of asteroidal impacts. Chronological, textural, and thermal constraints allow us to identify mete- orite impact breccias that formed during accretion (e.g., Kaidun), when asteroids were partly molten (e.g., mesosiderites), and during the subsequent disruption of asteroids (e.g., L chon- drites). Studies of chondrites including Kaidun suggest that chondrules accreted with similar- sized fragments of preexisting bodies that formed at greater heliocentric distances. In the inner solar system, chondrules appear to have been crucial for initiating accretion. Without chon- drules and rock fragments, dry dust failed to accrete to the nebular midplane because of nebular turbulence and spiraled into the protosun. The tiny mass of asteroids may be partly due to inefficient chondrule formation beyond 2 AU or less-efficient delivery of chondrules from near the protosun. 1. INTRODUCTION the asteroids had cooled, they were eroded by impacts for 4.5 G.y. or disrupted. However, this simple model is inad- Meteorites and returned asteroid samples are keys to equate as detailed petrologic studies and radiometric ages understanding the chemical, mineralogical, and physical suggest that the four stages overlapped in time.