Chapter 2 TEMPERATURES of AQUEOUS ALTERATION ON
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Presolar Grains As Tracers of Nebular and Parent Body Process- Ing of Chondritic Material
Workshop on Parent-Body and Nebular Modification of Chondritic Materials 4019.pdf PRESOLAR GRAINS AS TRACERS OF NEBULAR AND PARENT BODY PROCESS- ING OF CHONDRITIC MATERIAL. Gary R. Huss, Lunatic Asylum of the Charles Arms Laboratory, Division of Geological and Planetary Sciences, 170-25, California Institute of Tech- nology, Pasadena, CA 91125 Presolar grains such as diamond, SiC, These observations show that a single wide- graphite, and Al2O3 are present in the least al- spread reservoir of presolar grains was sam- tered members of all chondrite classes [1,2]. pled by all chondrite classes. This reservoir is These grains reside in the fine-grained “matrix” most plausibly the dust from the solar system’s and did not experience the chondrule-forming parent molecular cloud. process. Because most types of presolar grains The CI chondrite, Orgueil, and the matrices are chemically unstable in putative nebular of CM2 chondrites have the highest matrix- conditions and in chondritic meteorites, they normalized abundances of silicon carbide, and serve as sensitive monitors of nebular and par- graphite; have among the highest diamond ent-body conditions. abundances; and have diamonds with high ni- Tracers of Metamorphism: Isotopic and trogen contents and the highest contents of P3 trace element characteristics of some presolar noble gases [1-4]. These characteristics indi- material and the abundances of presolar grains cate that CI chondrites and the matrices of in “matrix” are functions of the metamorphic CM2 chondrites contain the least processed history of the host meteorite [1–4]. Currently sample of the presolar-grains reservoir from recognized presolar grains show a range of the sun’s parent molecular cloud. -

Brecciation and Chemical Heterogeneities of CI Chondrites
Geochimica et Cosmochimica Acta 70 (2006) 5371–5394 www.elsevier.com/locate/gca Brecciation and chemical heterogeneities of CI chondrites Andreas Morlok a,b,*, Addi Bischoff a, Thomas Stephan a, Christine Floss c, Ernst Zinner c, Elmar K. Jessberger a a Institut fu¨r Planetologie, Wilhelm-Klemm-Strasse 10, 48149 Mu¨nster, Germany b Department of Earth and Planetary Sciences, Faculty of Science, Kobe University, Kobe 657-8501, Japan c Laboratory for Space Sciences and Physics Department, Washington University, St. Louis, MO, USA Received 5 December 2005; accepted in revised form 3 August 2006 Abstract Fragments in the size range from 40 lm to several hundred lm in the CI chondrites Orgueil, Ivuna, Alais, and Tonk show a wide range of chemical compositions with variations in major elements such as iron (10.4–42.4 wt% FeO), silicon (12.7–42.2 wt% SiO2), and sulfur (1.01–15.8 wt% SO3), but also important minor elements such as phosphorous (up to 5.2 wt% P2O5) or calcium (up to 6.6 wt% CaO). These variations are the result of the varying mineralogical compositions of these fragments. The distribution of phyl- losilicates, magnetites, and possibly ferrihydrite, in particular, control the abundances of these elements. High REE contents—up to 150 times the solar abundances—were observed in phosphates, while matrix and sulfates are REE-depleted. The studied 113 fragments were subdivided into eight lithologies with similar mineralogical and thus chemical properties. The most common is the CGA lithology, consisting of a groundmass of Mg-rich, coarse-grained phyllosilicates and varying abundances of inclusions such as magnetite. -

The Early Geological History of the Moon Inferred from Ancient Lunar Meteorite Miller Range 13317
Meteoritics & Planetary Science 54, Nr 7, 1401–1430 (2019) doi: 10.1111/maps.13295 The early geological history of the Moon inferred from ancient lunar meteorite Miller Range 13317 N. M. CURRAN 1,2,*, K. H. JOY1, J. F. SNAPE3, J. F. PERNET-FISHER1, J. D. GILMOUR 1, A. A. NEMCHIN4, M. J. WHITEHOUSE3, and R. BURGESS1 1School of Earth and Environmental Sciences, University of Manchester, Oxford Road, Manchester M13 9PL, UK 2NASA Goddard Space Flight Center, 8800 Greenbelt Road, Greenbelt, Maryland 20771, USA 3Department of Geosciences, Swedish Museum of Natural History, SE-104 05 Stockholm, Sweden 4Department of Applied Geology, Curtin University, Perth, Western Australia 6845, Australia *Corresponding author. E-mail: [email protected] (Received 17 October 2018; revision accepted 14 March 2019) Abstract–Miller Range (MIL) 13317 is a heterogeneous basalt-bearing lunar regolith breccia that provides insights into the early magmatic history of the Moon. MIL 13317 is formed from a mixture of material with clasts having an affinity to Apollo ferroan anorthosites and basaltic volcanic rocks. Noble gas data indicate that MIL 13317 was consolidated into a breccia between 2610 Æ 780 Ma and 1570 Æ 470 Ma where it experienced a complex near- surface irradiation history for ~835 Æ 84 Myr, at an average depth of ~30 cm. The fusion crust has an intermediate composition (Al2O3 15.9 wt%; FeO 12.3 wt%) with an added incompatible trace element (Th 5.4 ppm) chemical component. Taking the fusion crust to be indicative of the bulk sample composition, this implies that MIL 13317 originated from a regolith that is associated with a mare-highland boundary that is KREEP-rich (i.e., K, rare earth elements, and P). -

The Effect of Aqueous Alteration and Metamorphism in the Survival of Presolar Silicate Grains in Chondrites
THE EFFECT OF AQUEOUS ALTERATION AND METAMORPHISM IN THE SURVIVAL OF PRESOLAR SILICATE GRAINS IN CHONDRITES Josep Mª Trigo-Rodriguez Institut of Space Sciences (CSIC), Campus UAB, Facultat de Ciències, Torre C5-parell-2ª, 08193 Bellaterra, Barcelona, Spain. E-mail: [email protected] Institut d’Estudis Espacials de Catalunya (IEEC), Edif.. Nexus, c/Gran Capità, 2-4, 08034 Barcelona, Spain and Jürgen Blum Institut fürGeophysik und extraterrestrische Physik, Technische Universität Braunschweig, Mendelssohnstr. 3, 38106 Braunschweig, Germany. E-mail: [email protected] ABSTRACT Relatively small amounts (typically between 2-200 parts per million) of presolar grains have been preserved in the matrices of chondritic meteorites. The measured abundances of the different types of grains are highly variable from one chondrite to another, but are higher in unequilibrated chondrites that have experienced little or no aqueous alteration and/or metamorphic heating than in processed meteorites. A general overview of the abundances measured in presolar grains (particularly the recently identified presolar silicates) contained in primitive chondrites is presented. Here we will focus on the most primitive chondrite groups, as typically the highest measured abundances of presolar grains occur in primitive chondrites that have experienced little thermal metamorphism. Looking at the most aqueously altered chondrite groups, we find a clear pattern of decreasing abundance of presolar silicate grains with increasing level of aqueous alteration. We conclude that the measured abundances of presolar grains in altered chondrites are strongly biased by their peculiar histories. Scales quantifying the intensity of aqueous alteration and shock metamorphism in chondrites could correlate with the content in presolar silicates. -

Finegrained Precursors Dominate the Micrometeorite Flux
Meteoritics & Planetary Science 47, Nr 4, 550–564 (2012) doi: 10.1111/j.1945-5100.2011.01292.x Fine-grained precursors dominate the micrometeorite flux Susan TAYLOR1*, Graciela MATRAJT2, and Yunbin GUAN3 1Cold Regions Research and Engineering Laboratory, 72 Lyme Road, Hanover, New Hampshire 03755–1290, USA 2University of Washington, Seattle, Washington 98105, USA 3Geological & Planetary Sciences MC 170-25, Caltech, Pasadena, California 91125, USA *Corresponding author. E-mail: [email protected] (Received 15 May 2011; revision accepted 22 September 2011) Abstract–We optically classified 5682 micrometeorites (MMs) from the 2000 South Pole collection into textural classes, imaged 2458 of these MMs with a scanning electron microscope, and made 200 elemental and eight isotopic measurements on those with unusual textures or relict phases. As textures provide information on both degree of heating and composition of MMs, we developed textural sequences that illustrate how fine-grained, coarse-grained, and single mineral MMs change with increased heating. We used this information to determine the percentage of matrix dominated to mineral dominated precursor materials (precursors) that produced the MMs. We find that at least 75% of the MMs in the collection derived from fine-grained precursors with compositions similar to CI and CM meteorites and consistent with dynamical models that indicate 85% of the mass influx of small particles to Earth comes from Jupiter family comets. A lower limit for ordinary chondrites is estimated at 2–8% based on MMs that contain Na-bearing plagioclase relicts. Less than 1% of the MMs have achondritic compositions, CAI components, or recognizable chondrules. Single mineral MMs often have magnetite zones around their peripheries. -

Cubanite & Associated Sulfides in CI Chondrites & Comet 81P/Wild 2
Cubanite & associated sulfides in CI chondrites & comet 81P/Wild 2: Implications for aqueous processing Eve L. Berger Thomas J. Zega Lindsay P. Keller Dante S. Lauretta Comets Workshop on Water in Asteroids ∠and Meteorites September 30, 2011 Characteristics of Stardust Sulfides Fe, Ni, Cu, Zn, Mn-bearing Mineralogy, Structure, Associations Modified by capture Unmodified by capture into aerogel into aerogel Nebular Formation Parent Body Precipitation via gas-solid processes Aqueous Modification on Wild 2 on other body Implications for the genesis of Wild 2 sulfides, source material(s), and mixing in the early Solar System as indicated by compositions, structures, and uniformity/heterogeneity of phases. Sulfide Populations Comet Wild 2 CI Chondrites Cubanite * * CuFe2S3 ✓ ✓ Pyrrhotite * * (Fe, Ni)1-xS ✓ ✓ Pentlandite * Minerals (Fe,Ni)9S8 ✓ ✓ Sphalerite * (Fe,Zn)S ✓ ? Cubanite * & Pyrrhotite ? ✓ Pyrrhotite * & Pentlandite ✓ ✓ Pyrrhotite * Assemblages & Sphalerite ✓ ? * Berger et al. (2011); Pentlandite: e.g., Bullock et al. (2005) CuFe2S3 Cubanite undergoes an irreversible phase transition at 210°C T ↑ to 210°C T ↓ to 210°C Cubanite Isocubanite CuFeS2 + Fe1-xS Low-T High-T orthorhombic cubic Caye et al. (2000), Miyamoto et al. (1980), Pruseth et al. (1999), Putnis et al. (1977) Stardust Cubanite C2054-5-26-1-16 Cu 100 nm Fe S [1 0 -2] 15.3 at.% Cu Low-temperature 35.0 at.% Fe orthorhombic cubanite never 49.7 at.% S experienced T > 210°C CI-chondrite Sulfides 2000µm Cubanite Cubanite & Pyrrhotite CI-chondrite Cubanite & Pyrrhotite CI-chondrite Cubanite -

Constraining the Evolutionary History of the Moon and the Inner Solar System: a Case for New Returned Lunar Samples
Space Sci Rev (2019) 215:54 https://doi.org/10.1007/s11214-019-0622-x Constraining the Evolutionary History of the Moon and the Inner Solar System: A Case for New Returned Lunar Samples Romain Tartèse1 · Mahesh Anand2,3 · Jérôme Gattacceca4 · Katherine H. Joy1 · James I. Mortimer2 · John F. Pernet-Fisher1 · Sara Russell3 · Joshua F. Snape5 · Benjamin P. Weiss6 Received: 23 August 2019 / Accepted: 25 November 2019 / Published online: 2 December 2019 © The Author(s) 2019 Abstract The Moon is the only planetary body other than the Earth for which samples have been collected in situ by humans and robotic missions and returned to Earth. Scien- tific investigations of the first lunar samples returned by the Apollo 11 astronauts 50 years ago transformed the way we think most planetary bodies form and evolve. Identification of anorthositic clasts in Apollo 11 samples led to the formulation of the magma ocean concept, and by extension the idea that the Moon experienced large-scale melting and differentiation. This concept of magma oceans would soon be applied to other terrestrial planets and large asteroidal bodies. Dating of basaltic fragments returned from the Moon also showed that a relatively small planetary body could sustain volcanic activity for more than a billion years after its formation. Finally, studies of the lunar regolith showed that in addition to contain- ing a treasure trove of the Moon’s history, it also provided us with a rich archive of the past 4.5 billion years of evolution of the inner Solar System. Further investigations of samples returned from the Moon over the past five decades led to many additional discoveries, but also raised new and fundamental questions that are difficult to address with currently avail- able samples, such as those related to the age of the Moon, duration of lunar volcanism, the Role of Sample Return in Addressing Major Questions in Planetary Sciences Edited by Mahesh Anand, Sara Russell, Yangting Lin, Meenakshi Wadhwa, Kuljeet Kaur Marhas and Shogo Tachibana B R. -

Ancient Martian Life in Allan Hills 84001? Status of Some Current Controversies
Workshop on the Issue of Martian Meteorites 7043.pdf ANCIENT MARTIAN LIFE IN ALLAN HILLS 84001? STATUS OF SOME CURRENT CONTROVERSIES. A. H. Treiman, Lunar and Planetary Institute, 3600 Bay Area Boulevard, Houston TX 77058, USA ([email protected]). Four lines of evidence were taken to suggest that ALH Also, it is possible that the elongate magnetites formed at 84001 contained traces of ancient martian life preserved in high T, but after the carbonates formed [5]. Magnetites are its carbonate mineral masses [1]: abundance of organic also present in void spaces in the carbonate, either as the compounds (PAHs), disequilibrium mineral assemblages, product of decarbonation at high T [21] or as entrapped from morphology of sub-micron magnetite crystals, and presence the aqueous fluid that deposited the carbonates [22]. of objects comparable in size and shape to bacteria. This Summary. There is no consensus on the formation T or evidence is predicated on the carbonate globules having mechanism of carbonate masses. The bulk of evidence, in formed at temperatures conducive to life. Here, I review my opinion, is not consistent with T>400°C [4,6,7]. At pres- evidence on carbonate formation temperature, martian ori- ent, there seems no way to tell whether the carbonates gin of organic compounds, and bacteria-shaped objects. formed at moderate or low T: hotter or colder than ~150°C. Formation Temperature: A precondition for signs of Organic Matter: McKay et al. [1] presented evidence life associated with the carbonate masses [1] is that the that the carbonate pancakes in ALH 84001 are enriched in masses formed at T amenable to life (as we know it) [2], polycyclic aromatic hydrocarbons, PAHs, which are organic <~150°C. -

Lpi Summer Intern Program in Planetary Science
LPI SUMMER INTERN PROGRAM IN PLANETARY SCIENCE Papers Presented at the August 11, 2011 — Houston, Texas Papers Presented at the Twenty-Seventh Annual Summer Intern Conference August 11, 2011 Houston, Texas 2011 Summer Intern Program for Undergraduates Lunar and Planetary Institute Sponsored by Lunar and Planetary Institute NASA Johnson Space Center Compiled in 2011 by Meeting and Publication Services Lunar and Planetary Institute USRA Houston 3600 Bay Area Boulevard, Houston TX 77058-1113 The Lunar and Planetary Institute is operated by the Universities Space Research Association under a cooperative agreement with the Science Mission Directorate of the National Aeronautics and Space Administration. Any opinions, findings, and conclusions or recommendations expressed in this volume are those of the author(s) and do not necessarily reflect the views of the National Aeronautics and Space Administration. Material in this volume may be copied without restraint for library, abstract service, education, or personal research purposes; however, republication of any paper or portion thereof requires the written permission of the authors as well as the appropriate acknowledgment of this publication. 2011 Intern Conference iii HIGHLIGHTS Special Activities June 6, 2011 Tour of the Stardust Lab and Lunar Curatorial Facility JSC July 22, 2011 Tour of the Meteorite Lab JSC August 4, 2011 NASA VIP Tour JSC/ NASA Johnson Space Center and Sonny Carter SCTF Training Facility Site Visit Intern Brown Bag Seminars Date Speaker Topic Location June 8, 2011 Paul -

(2000) Forging Asteroid-Meteorite Relationships Through Reflectance
Forging Asteroid-Meteorite Relationships through Reflectance Spectroscopy by Thomas H. Burbine Jr. B.S. Physics Rensselaer Polytechnic Institute, 1988 M.S. Geology and Planetary Science University of Pittsburgh, 1991 SUBMITTED TO THE DEPARTMENT OF EARTH, ATMOSPHERIC, AND PLANETARY SCIENCES IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN PLANETARY SCIENCES AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY FEBRUARY 2000 © 2000 Massachusetts Institute of Technology. All rights reserved. Signature of Author: Department of Earth, Atmospheric, and Planetary Sciences December 30, 1999 Certified by: Richard P. Binzel Professor of Earth, Atmospheric, and Planetary Sciences Thesis Supervisor Accepted by: Ronald G. Prinn MASSACHUSES INSTMUTE Professor of Earth, Atmospheric, and Planetary Sciences Department Head JA N 0 1 2000 ARCHIVES LIBRARIES I 3 Forging Asteroid-Meteorite Relationships through Reflectance Spectroscopy by Thomas H. Burbine Jr. Submitted to the Department of Earth, Atmospheric, and Planetary Sciences on December 30, 1999 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Planetary Sciences ABSTRACT Near-infrared spectra (-0.90 to ~1.65 microns) were obtained for 196 main-belt and near-Earth asteroids to determine plausible meteorite parent bodies. These spectra, when coupled with previously obtained visible data, allow for a better determination of asteroid mineralogies. Over half of the observed objects have estimated diameters less than 20 k-m. Many important results were obtained concerning the compositional structure of the asteroid belt. A number of small objects near asteroid 4 Vesta were found to have near-infrared spectra similar to the eucrite and howardite meteorites, which are believed to be derived from Vesta. -
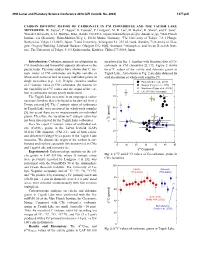
CARBON ISOTOPIC RATIOS of CARBONATE in CM CHONDRITES and the TAGISH LAKE METEORITE. W. Fujiya1, P. Hoppe2, K. Fukuda3, P. Lindgren4, M
49th Lunar and Planetary Science Conference 2018 (LPI Contrib. No. 2083) 1377.pdf CARBON ISOTOPIC RATIOS OF CARBONATE IN CM CHONDRITES AND THE TAGISH LAKE METEORITE. W. Fujiya1, P. Hoppe2, K. Fukuda3, P. Lindgren4, M. R. Lee5, M. Koike6, K. Shirai6, and Y. Sano6. 1Ibaraki University, 2-1-1 Bunkyo, Mito, Ibaraki 310-8512, Japan ([email protected]), 2Max Planck Institute for Chemistry, Hahn-Meitner-Weg 1, 55128 Mainz, Germany, 3The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan, 4Lund University, Sölvegatan 12, 223 62 Lund, Sweden, 5University of Glas- gow, Gregory Building, Lilybank Gardens, Glasgow G12 8QQ, Scotland, 6Atmosphere and Ocean Research Insti- tute, The University of Tokyo, 5-1-5 Kashiwanoha, Kashiwa, Chiba 277-8564, Japan. Introduction: Carbonate minerals are ubiquitous in are plotted in Fig. 1, together with literature data of Ca- CM chondrites and formed by aqueous alteration in the carbonate in CM chondrites [2,3,7]. Figure 2 shows parent body. Previous studies have shown that C iso- the13C values of the calcite and dolomite grains in topic ratios of CM carbonates are highly variable at Tagish Lake. Also shown in Fig. 2 are data obtained by whole-rock scales as well as among individual grains in acid dissolution of whole-rock samples [5]. single meteorites [e.g., 1-3]. Despite extensive studies Paris (Vacher et al., 2017) on C isotopic ratios of CM carbonates, the reasons for 100 Nogoya (Fujiya et al., 2016) the variability in 13C values and the origin of the car- Murchison (Fujiya et al., 2015) bon in carbonates remain poorly understood. -

Chondrules Reveal Large-Scale Outward Transport of Inner Solar System Materials in the Protoplanetary Disk
Chondrules reveal large-scale outward transport of inner Solar System materials in the protoplanetary disk Curtis D. Williamsa,1, Matthew E. Sanborna, Céline Defouilloyb,2, Qing-Zhu Yina,1, Noriko T. Kitab, Denton S. Ebelc, Akane Yamakawaa,3, and Katsuyuki Yamashitad aDepartment of Earth and Planetary Sciences, University of California, Davis, CA 95616; bWiscSIMS, Department of Geoscience, University of Wisconsin–Madison, Madison, WI 53706; cDepartment of Earth and Planetary Sciences, American Museum of Natural History, New York, NY 10024; and dGraduate School of Natural Science and Technology, Okayama University, Kita-ku, 700-8530 Okayama, Japan Edited by H. J. Melosh, Purdue University, West Lafayette, IN, and approved August 9, 2020 (received for review March 19, 2020) Dynamic models of the protoplanetary disk indicate there should actually be composed of solids with diverse formation histories be large-scale material transport in and out of the inner Solar Sys- and, thus, distinct isotope signatures, which can only be revealed tem, but direct evidence for such transport is scarce. Here we show by studying individual components in primitive chondritic me- that the e50Ti-e54Cr-Δ17O systematics of large individual chon- teorites (e.g., chondrules). Chondrules are millimeter-sized drules, which typically formed 2 to 3 My after the formation of spherules that evolved as free-floating objects processed by the first solids in the Solar System, indicate certain meteorites (CV transient heating in the protoplanetary disk (1) and represent a and CK chondrites) that formed in the outer Solar System accreted major solid component (by volume) of the disk that is accreted an assortment of both inner and outer Solar System materials, as into most chondrites.