Tagish Lake Meteorite Are Identifying Carbon Allotropes (14), and Electron Intermediate Between CM and CI Meteorites
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Presolar Grains As Tracers of Nebular and Parent Body Process- Ing of Chondritic Material
Workshop on Parent-Body and Nebular Modification of Chondritic Materials 4019.pdf PRESOLAR GRAINS AS TRACERS OF NEBULAR AND PARENT BODY PROCESS- ING OF CHONDRITIC MATERIAL. Gary R. Huss, Lunatic Asylum of the Charles Arms Laboratory, Division of Geological and Planetary Sciences, 170-25, California Institute of Tech- nology, Pasadena, CA 91125 Presolar grains such as diamond, SiC, These observations show that a single wide- graphite, and Al2O3 are present in the least al- spread reservoir of presolar grains was sam- tered members of all chondrite classes [1,2]. pled by all chondrite classes. This reservoir is These grains reside in the fine-grained “matrix” most plausibly the dust from the solar system’s and did not experience the chondrule-forming parent molecular cloud. process. Because most types of presolar grains The CI chondrite, Orgueil, and the matrices are chemically unstable in putative nebular of CM2 chondrites have the highest matrix- conditions and in chondritic meteorites, they normalized abundances of silicon carbide, and serve as sensitive monitors of nebular and par- graphite; have among the highest diamond ent-body conditions. abundances; and have diamonds with high ni- Tracers of Metamorphism: Isotopic and trogen contents and the highest contents of P3 trace element characteristics of some presolar noble gases [1-4]. These characteristics indi- material and the abundances of presolar grains cate that CI chondrites and the matrices of in “matrix” are functions of the metamorphic CM2 chondrites contain the least processed history of the host meteorite [1–4]. Currently sample of the presolar-grains reservoir from recognized presolar grains show a range of the sun’s parent molecular cloud. -

FNESS Strategic Plan
Strategic Plan 2013-2015 At a Glance FNESS evolved from the Society of Native Indian Fire Fighters of BC (SNIFF), which was established in 1986. SNIFF’s initial objectives were to help reduce the number of fire-related deaths on First Nations reserves, but it changed its emphasis to incorporate a greater spectrum of emergency services. In 1994, SNIFF changed its name to First Nations’ Emergency Services Society of BC to reflect the growing diversity of services it provides. Today our organization continues to gain recognition and trust within First Nations communities and within Aboriginal Affairs and Northern Development Canada (AANDC) and other organizations. This is reflected in both the growing demand of service requests from First Nations communities and the development of more government-sponsored programs with FNESS. r e v Ri k e s l A Inset 1 Tagish Lake Teslin 1059 Daylu Dena Atlin Lake 501 Taku River Tlingit r e v Liard Atlin Lake i R River ku 504 Dease River K Fort a e Nelson T r t 594 Ts'kw'aylaxw e c iv h R ik River 686 Bonaparte a se a 687 Skeetchestn e D Fort Nelson R i v e First Nations in 543 Fort Nelson Dease r 685 Ashcroft Lake Dease Lake 592 Xaxli'p British Columbia 593 T'it'q'et 544 Prophet River 591 Cayoose Creek 692 Oregon Jack Creek 682 Tahltan er 683 Iskut a Riv kw r s e M u iv R Finlay F R Scale ra e n iv s i er 610 Kwadacha k e i r t 0 75 150 300 Km S 694 Cook's Ferry Thutade R r Tatlatui Lake i e 609 Tsay Keh Dene v Iskut iv 547 Blueberry River e R Lake r 546 Halfway River 548 Doig River 698 Shackan Location -

Magnetite Biomineralization and Ancient Life on Mars Richard B Frankel* and Peter R Buseckt
Magnetite biomineralization and ancient life on Mars Richard B Frankel* and Peter R Buseckt Certain chemical and mineral features of the Martian meteorite with a mass distribution unlike terrestrial PAHs or those from ALH84001 were reported in 1996 to be probable evidence of other meteorites; thirdly, bacterium-shaped objects (BSOs) ancient life on Mars. In spite of new observations and up to several hundred nanometers long that resemble fos interpretations, the question of ancient life on Mars remains silized terrestrial microorganisms; and lastly, 10-100 nm unresolved. Putative biogenic, nanometer magnetite has now magnetite (Fe304), pyrrhotite (Fel_xS), and greigite (Fe3S4) become a leading focus in the debate. crystals. These minerals were cited as evidence because of their similarity to biogenic magnetic minerals in terrestrial Addresses magnetotactic bacteria. *Department of Physics, California Polytechnic State University, San Luis Obispo, California 93407, USA; e-mail: [email protected] The ancient life on Mars hypothesis has been extensively tDepartments of Geology and Chemistry/Biochemistry, Arizona State challenged, and alternative non-biological processes have University, Tempe, Arizona 85287-1404, USA; e-mail: [email protected] been proposed for each of the four features cited by McKay et al. [4]. In this paper we review the current situa tion regarding their proposed evidence, focusing on the Abbreviations putative biogenic magnetite crystals. BCM biologically controlled mineralization BIM biologically induced mineralization BSO bacterium-shaped object Evidence for and against ancient Martian life PAH polycyclic aromatic hydrocarbon PAHs and BSOs Reports of contamination by terrestrial organic materials [5°,6°] and the similarity of ALH84001 PAHs to non-bio genic PAHs in carbonaceous chondrites [7,8] make it Introduction difficult to positively identify PAHs of non-terrestrial, bio A 2 kg carbonaceous stony meteorite, designated genic origin. -

Brecciation and Chemical Heterogeneities of CI Chondrites
Geochimica et Cosmochimica Acta 70 (2006) 5371–5394 www.elsevier.com/locate/gca Brecciation and chemical heterogeneities of CI chondrites Andreas Morlok a,b,*, Addi Bischoff a, Thomas Stephan a, Christine Floss c, Ernst Zinner c, Elmar K. Jessberger a a Institut fu¨r Planetologie, Wilhelm-Klemm-Strasse 10, 48149 Mu¨nster, Germany b Department of Earth and Planetary Sciences, Faculty of Science, Kobe University, Kobe 657-8501, Japan c Laboratory for Space Sciences and Physics Department, Washington University, St. Louis, MO, USA Received 5 December 2005; accepted in revised form 3 August 2006 Abstract Fragments in the size range from 40 lm to several hundred lm in the CI chondrites Orgueil, Ivuna, Alais, and Tonk show a wide range of chemical compositions with variations in major elements such as iron (10.4–42.4 wt% FeO), silicon (12.7–42.2 wt% SiO2), and sulfur (1.01–15.8 wt% SO3), but also important minor elements such as phosphorous (up to 5.2 wt% P2O5) or calcium (up to 6.6 wt% CaO). These variations are the result of the varying mineralogical compositions of these fragments. The distribution of phyl- losilicates, magnetites, and possibly ferrihydrite, in particular, control the abundances of these elements. High REE contents—up to 150 times the solar abundances—were observed in phosphates, while matrix and sulfates are REE-depleted. The studied 113 fragments were subdivided into eight lithologies with similar mineralogical and thus chemical properties. The most common is the CGA lithology, consisting of a groundmass of Mg-rich, coarse-grained phyllosilicates and varying abundances of inclusions such as magnetite. -

Lost Lake by Robert Verish
Meteorite-Times Magazine Contents by Editor Like Sign Up to see what your friends like. Featured Monthly Articles Accretion Desk by Martin Horejsi Jim’s Fragments by Jim Tobin Meteorite Market Trends by Michael Blood Bob’s Findings by Robert Verish IMCA Insights by The IMCA Team Micro Visions by John Kashuba Galactic Lore by Mike Gilmer Meteorite Calendar by Anne Black Meteorite of the Month by Michael Johnson Tektite of the Month by Editor Terms Of Use Materials contained in and linked to from this website do not necessarily reflect the views or opinions of The Meteorite Exchange, Inc., nor those of any person connected therewith. In no event shall The Meteorite Exchange, Inc. be responsible for, nor liable for, exposure to any such material in any form by any person or persons, whether written, graphic, audio or otherwise, presented on this or by any other website, web page or other cyber location linked to from this website. The Meteorite Exchange, Inc. does not endorse, edit nor hold any copyright interest in any material found on any website, web page or other cyber location linked to from this website. The Meteorite Exchange, Inc. shall not be held liable for any misinformation by any author, dealer and or seller. In no event will The Meteorite Exchange, Inc. be liable for any damages, including any loss of profits, lost savings, or any other commercial damage, including but not limited to special, consequential, or other damages arising out of this service. © Copyright 2002–2010 The Meteorite Exchange, Inc. All rights reserved. No reproduction of copyrighted material is allowed by any means without prior written permission of the copyright owner. -

March 21–25, 2016
FORTY-SEVENTH LUNAR AND PLANETARY SCIENCE CONFERENCE PROGRAM OF TECHNICAL SESSIONS MARCH 21–25, 2016 The Woodlands Waterway Marriott Hotel and Convention Center The Woodlands, Texas INSTITUTIONAL SUPPORT Universities Space Research Association Lunar and Planetary Institute National Aeronautics and Space Administration CONFERENCE CO-CHAIRS Stephen Mackwell, Lunar and Planetary Institute Eileen Stansbery, NASA Johnson Space Center PROGRAM COMMITTEE CHAIRS David Draper, NASA Johnson Space Center Walter Kiefer, Lunar and Planetary Institute PROGRAM COMMITTEE P. Doug Archer, NASA Johnson Space Center Nicolas LeCorvec, Lunar and Planetary Institute Katherine Bermingham, University of Maryland Yo Matsubara, Smithsonian Institute Janice Bishop, SETI and NASA Ames Research Center Francis McCubbin, NASA Johnson Space Center Jeremy Boyce, University of California, Los Angeles Andrew Needham, Carnegie Institution of Washington Lisa Danielson, NASA Johnson Space Center Lan-Anh Nguyen, NASA Johnson Space Center Deepak Dhingra, University of Idaho Paul Niles, NASA Johnson Space Center Stephen Elardo, Carnegie Institution of Washington Dorothy Oehler, NASA Johnson Space Center Marc Fries, NASA Johnson Space Center D. Alex Patthoff, Jet Propulsion Laboratory Cyrena Goodrich, Lunar and Planetary Institute Elizabeth Rampe, Aerodyne Industries, Jacobs JETS at John Gruener, NASA Johnson Space Center NASA Johnson Space Center Justin Hagerty, U.S. Geological Survey Carol Raymond, Jet Propulsion Laboratory Lindsay Hays, Jet Propulsion Laboratory Paul Schenk, -

Organic Matter in Meteorites Department of Inorganic Chemistry, University of Barcelona, Spain
REVIEW ARTICLE INTERNATIONAL MICROBIOLOGY (2004) 7:239-248 www.im.microbios.org Jordi Llorca Organic matter in meteorites Department of Inorganic Chemistry, University of Barcelona, Spain Summary. Some primitive meteorites are carbon-rich objects containing a vari- ety of organic molecules that constitute a valuable record of organic chemical evo- lution in the universe prior to the appearance of microorganisms. Families of com- pounds include hydrocarbons, alcohols, aldehydes, ketones, carboxylic acids, amino acids, amines, amides, heterocycles, phosphonic acids, sulfonic acids, sugar-relat- ed compounds and poorly defined high-molecular weight macromolecules. A vari- ety of environments are required in order to explain this organic inventory, includ- ing interstellar processes, gas-grain reactions operating in the solar nebula, and hydrothermal alteration of parent bodies. Most likely, substantial amounts of such Received 15 September 2004 organic materials were delivered to the Earth via a late accretion, thereby provid- Accepted 15 October 2004 ing organic compounds important for the emergence of life itself, or that served as a feedstock for further chemical evolution. This review discusses the organic con- Address for correspondence: Departament de Química Inorgànica tent of primitive meteorites and their relevance to the build up of biomolecules. Universitat de Barcelona [Int Microbiol 2004; 7(4):239-248] Martí i Franquès, 1-11 08028 Barcelona, Spain Tel. +34-934021235. Fax +34-934907725 Key words: primitive meteorites · prebiotic chemistry · chemical evolution · E-mail: [email protected] origin of life providing new opportunities for scientific advancement. One Introduction of the most important findings regarding such bodies is that comets and certain types of meteorites contain organic mole- Like a carpentry shop littered with wood shavings after the cules formed in space that may have had a relevant role in the work is done, debris left over from the formation of the Sun origin of the first microorganisms on Earth. -
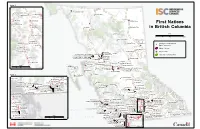
For a Larger Version of the First Nations in British
#! Inset 1 Tagish Lake #! Teslin 502 Liard Atlin Lake #!501 Taku River Tlingit L 594 Ts'kw 'aylaxw iard #! Atlin Lake R 687 Skeetchestn ive #! ! 504 Dease River K r 686 Bonaparte # #! e r t e c iv h R ik #! a se a e D Fort Nelson R ! i # ! 592 Xaxli'p #! 685 Ashcroft v # e 543 Fort Nelson Dease r #! 593 T'it'q'et Lake Dease Lake #! First Nations 591 Cayoose Creek #! 692 Oregon Jack Creek 682 Tahltan #! 544 P rophet River r #! a ive in British Colum bia F R in British Colum bia 683 Iskut r #! kw a r s s e M u e iv r R Finlay R e iv n er i 610 Kw ad acha k Scale i t #! ! S R # 694 Cook's Ferry i v 0 75 150 300 km e r Thutade r e Lake I iv Tatlatui 609 Tsay Keh Dene skut R #! 547 Blueberry River Lake #! 698 Shackan #! #! #! #! 696 Nicom en 546 Halfw ay River 548 Doig River 705 Lytton #! #! Location of First Nation's 699 Nooaitch Main Community #! Williston Fort St John 707 Skuppah #! Lake N Indian Reserve a ! s 542 Saulteau # 706 Siska s #! #! 704 Kanaka Bar #! R Takla i 545 W est Moberly v City or Town e Lake r 532 Kispiox 533 Glen Vow ell 608 Takla 677 Nisga'a Village of New Aiyansh 537 Gitanyow 531 Gitanm aax #! #! Park and Protected Area 679 Nisga'a Village of Gitw inksihlkw #! #!!534 Hagw ilget 678 Nisga'a Village of Laxgalt'sap #!#! # #! 700 Boothroyd ! #! #! 535 Gitsegukla 671 Nisga'a Village of Gingolx#! # ! Babine #! 618 McLeod Lake 536 Gitw ar ngak # e 530 W itset v i Sm ithers 674 Lax Kw 'alaam s R Lake 617 Tl'azt'en ! 701 Boston Bar ! # #! Terrace #!680 Kitselas 728 Yekooche ! # #! # #! 730 Binche W hut'en 673 Metlakatla ena -

Meteoroids: the Smallest Solar System Bodies
Passage of Bolides through the Atmosphere 1 O. Popova Abstract Different fragmentation models are applied to a number of events, including the entry of TC3 2008 asteroid in order to reproduce existing observational data. keywords meteoroid entry · fragmentation · modeling 1 Introduction Fragmentation is a very important phenomenon which occurs during the meteoroid entry into the atmosphere and adds more drastic effects than mere deceleration and ablation. Modeling of bolide 6 fragmentation (100 – 10 kg in mass) may be divided into several approaches. Detail fitting of observational data (deceleration and/or light curves) allows the determination of some meteoroid parameters (ablation and shape-density coefficients, fragmentation points, amount of mass loss) (Ceplecha et al. 1993; Ceplecha and ReVelle 2005). Observational data with high accuracy are needed for the gross-fragmentation model (Ceplecha et al. 1993), which is used for the analysis of European and Desert bolide networks data. Hydrodynamical models, which describe the entry of the meteoroid 6 including evolution of its material, are applied mainly for large bodies (>10 kg) (Boslough et al. 1994; Svetsov et al. 1995; Shuvalov and Artemieva 2002, and others). Numerous papers were devoted to the application of standard equations for large meteoroid entry in the attempts to reproduce dynamics and/or radiation for different bolides and to predict meteorite falls. These modeling efforts are often supplemented by different fragmentation models (Baldwin and Sheaffer, 1971; Borovi6ka et al. 1998; Artemieva and Shuvalov, 2001; Bland and Artemieva, 2006, and others). The fragmentation may occur in different ways. For example, few large fragments are formed. These pieces initially interact through their shock waves and then continue their flight independently. -

The Early Geological History of the Moon Inferred from Ancient Lunar Meteorite Miller Range 13317
Meteoritics & Planetary Science 54, Nr 7, 1401–1430 (2019) doi: 10.1111/maps.13295 The early geological history of the Moon inferred from ancient lunar meteorite Miller Range 13317 N. M. CURRAN 1,2,*, K. H. JOY1, J. F. SNAPE3, J. F. PERNET-FISHER1, J. D. GILMOUR 1, A. A. NEMCHIN4, M. J. WHITEHOUSE3, and R. BURGESS1 1School of Earth and Environmental Sciences, University of Manchester, Oxford Road, Manchester M13 9PL, UK 2NASA Goddard Space Flight Center, 8800 Greenbelt Road, Greenbelt, Maryland 20771, USA 3Department of Geosciences, Swedish Museum of Natural History, SE-104 05 Stockholm, Sweden 4Department of Applied Geology, Curtin University, Perth, Western Australia 6845, Australia *Corresponding author. E-mail: [email protected] (Received 17 October 2018; revision accepted 14 March 2019) Abstract–Miller Range (MIL) 13317 is a heterogeneous basalt-bearing lunar regolith breccia that provides insights into the early magmatic history of the Moon. MIL 13317 is formed from a mixture of material with clasts having an affinity to Apollo ferroan anorthosites and basaltic volcanic rocks. Noble gas data indicate that MIL 13317 was consolidated into a breccia between 2610 Æ 780 Ma and 1570 Æ 470 Ma where it experienced a complex near- surface irradiation history for ~835 Æ 84 Myr, at an average depth of ~30 cm. The fusion crust has an intermediate composition (Al2O3 15.9 wt%; FeO 12.3 wt%) with an added incompatible trace element (Th 5.4 ppm) chemical component. Taking the fusion crust to be indicative of the bulk sample composition, this implies that MIL 13317 originated from a regolith that is associated with a mare-highland boundary that is KREEP-rich (i.e., K, rare earth elements, and P). -

The Effect of Aqueous Alteration and Metamorphism in the Survival of Presolar Silicate Grains in Chondrites
THE EFFECT OF AQUEOUS ALTERATION AND METAMORPHISM IN THE SURVIVAL OF PRESOLAR SILICATE GRAINS IN CHONDRITES Josep Mª Trigo-Rodriguez Institut of Space Sciences (CSIC), Campus UAB, Facultat de Ciències, Torre C5-parell-2ª, 08193 Bellaterra, Barcelona, Spain. E-mail: [email protected] Institut d’Estudis Espacials de Catalunya (IEEC), Edif.. Nexus, c/Gran Capità, 2-4, 08034 Barcelona, Spain and Jürgen Blum Institut fürGeophysik und extraterrestrische Physik, Technische Universität Braunschweig, Mendelssohnstr. 3, 38106 Braunschweig, Germany. E-mail: [email protected] ABSTRACT Relatively small amounts (typically between 2-200 parts per million) of presolar grains have been preserved in the matrices of chondritic meteorites. The measured abundances of the different types of grains are highly variable from one chondrite to another, but are higher in unequilibrated chondrites that have experienced little or no aqueous alteration and/or metamorphic heating than in processed meteorites. A general overview of the abundances measured in presolar grains (particularly the recently identified presolar silicates) contained in primitive chondrites is presented. Here we will focus on the most primitive chondrite groups, as typically the highest measured abundances of presolar grains occur in primitive chondrites that have experienced little thermal metamorphism. Looking at the most aqueously altered chondrite groups, we find a clear pattern of decreasing abundance of presolar silicate grains with increasing level of aqueous alteration. We conclude that the measured abundances of presolar grains in altered chondrites are strongly biased by their peculiar histories. Scales quantifying the intensity of aqueous alteration and shock metamorphism in chondrites could correlate with the content in presolar silicates. -

Processing of Meteoritic Organic Materials As a Possible Analog of Early Molecular Evolution in Planetary Environments
Processing of meteoritic organic materials as a possible analog of early molecular evolution in planetary environments Sandra Pizzarelloa,1, Stephen K. Davidowskia, Gregory P. Hollanda, and Lynda B. Williamsb aDepartment of Chemistry and Biochemistry, Arizona State University, Tempe, AZ 85287-1604; and bSchool of Earth and Space Exploration, Arizona State University, Tempe, AZ 85287-1404 Edited* by Jonathan I. Lunine, Cornell University, Ithaca, NY, and approved August 2, 2013 (received for review May 14, 2013) The composition of the Sutter’s Mill meteorite insoluble organic hydrocarbons; several of these have identical counterparts in the material was studied both in toto by solid-state NMR spectroscopy terrestrial biosphere and have been extensively studied and of the powders and by gas chromatography–mass spectrometry reviewed (2). The IOM represents the larger portion of CC or- analyses of compounds released upon their hydrothermal treat- ganic carbon, up to 99%, and is not known in molecular detail. It ment. Results were compared with those obtained for other mete- is often referred to as kerogen-like because, like kerogen, it is orites of diverse classifications (Murray, GRA 95229, Murchison, insoluble and isolated after dissolution of free compounds and Orgueil, and Tagish Lake) and found to be so far unique in regard minerals by repeated washes with strong acids (4). The IOM bulk to the molecular species released. These include, in addition to O- composition can be only inferred from spectroscopy, e.g., NMR containing aromatic compounds, complex polyether- and ester- and infrared, or decomposition studies, which have suggested containing alkyl molecules of prebiotic appeal and never detected a complex macromolecular structure with both aromatic and in meteorites before.