Dimethyl Fumarate for Plaque Psoriasis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Left/Right Comparison of Twice-Daily Calcipotriol Ointment and Calcitriol Ointment in Patients with Psoriasis: the Effect on Keratinocyte Subpopulations
Acta Derm Venereol 2004; 84: 195–200 INVESTIGATIVE REPORT A Left/Right Comparison of Twice-Daily Calcipotriol Ointment and Calcitriol Ointment in Patients with Psoriasis: The Effect on Keratinocyte Subpopulations Mannon E.J. FRANSSEN, Gys J. DE JONGH, Piet E.J. VAN ERP and Peter C.M. VAN DE KERKHOF Department of Dermatology, University Medical Centre Nijmegen, The Netherlands Vitamin D3 analogues are a first-line treatment of Calcipotriol (Daivonex1,50mg/g ointment, Leo chronic plaque psoriasis, but so far, comparative clinical Pharmaceutical Products, Denmark) has been investi- studies on calcipotriol and calcitriol ointment are sparse, gated intensively during the last decade, and has proven and in particular no comparative studies are available on to be a valuable tool in the management of chronic cell biological effects of these compounds in vivo. Using plaque psoriasis. A review by Ashcroft et al. (1), based on flow cytometric assessment, we investigated whether these a large number of randomized controlled trials, showed compounds had different effects on the composition and that calcipotriol was at least as effective as potent DNA synthesis of epidermal cell populations responsible topical corticosteroids, 1a,-25-dihydroxycholecalciferol for the psoriatic phenotype. For 8 weeks, 20 patients with (calcitriol), short-contact dithranol, tacalcitol and coal psoriasis vulgaris were treated twice daily with calcipo- tar. Recently, Scott et al. (2) presented an overview of triol and calcitriol ointment in a left/right comparative studies on the use of calcipotriol ointment in the study. Before and after treatment, clinical assessment of management of psoriasis. They reconfirmed the super- target lesions was performed, together with flow cyto- ior efficacy of a twice-daily calcipotriol ointment metric analysis of epidermal subpopulations with respect regimen to the treatments as mentioned above, and to keratin (K) 10, K6, vimentin and DNA distribution. -

Phototoxicity of 7-Oxycoumarins with Keratinocytes in Culture T ⁎ Christophe Guillona, , Yi-Hua Janb, Diane E
Bioorganic Chemistry 89 (2019) 103014 Contents lists available at ScienceDirect Bioorganic Chemistry journal homepage: www.elsevier.com/locate/bioorg Phototoxicity of 7-oxycoumarins with keratinocytes in culture T ⁎ Christophe Guillona, , Yi-Hua Janb, Diane E. Heckc, Thomas M. Marianob, Robert D. Rappa, Michele Jettera, Keith Kardosa, Marilyn Whittemored, Eric Akyeaa, Ivan Jabine, Jeffrey D. Laskinb, Ned D. Heindela a Department of Chemistry, Lehigh University, Bethlehem, PA 18015, USA b Department of Environmental and Occupational Health, Rutgers University School of Public Health, Piscataway, NJ 08854, USA c Department of Environmental Science, New York Medical College, Valhalla, NY 10595, USA d Buckman Laboratories, 1256 N. McLean Blvd, Memphis, TN 38108, USA e Laboratoire de Chimie Organique, Université Libre de Bruxelles, B-1050 Brussels, Belgium ARTICLE INFO ABSTRACT Keywords: Seventy-one 7-oxycoumarins, 66 synthesized and 5 commercially sourced, were tested for their ability to inhibit 7-hydroxycoumarins growth in murine PAM212 keratinocytes. Forty-nine compounds from the library demonstrated light-induced 7-oxycoumarins lethality. None was toxic in the absence of UVA light. Structure-activity correlations indicate that the ability of Furocoumarins the compounds to inhibit cell growth was dependent not only on their physiochemical characteristics, but also Psoralens on their ability to absorb UVA light. Relative lipophilicity was an important factor as was electron density in the Methoxsalen pyrone ring. Coumarins with electron withdrawing moieties – cyano and fluoro at C – were considerably less 8-MOP 3 Phototoxicity active while those with bromines or iodine at that location displayed enhanced activity. Coumarins that were PAM212 keratinocytes found to inhibit keratinocyte growth were also tested for photo-induced DNA plasmid nicking. -

Profile of Clinical Efficacy and Safety of Topical Tacalcitol
leone 9-06-2005 14:07 Pagina 13 ACTA BIO MED 2005; 76; 13-19 © Mattioli 1885 U PDATE Profile of clinical efficacy and safety of topical tacalcitol Giovanni Leone, Alessia Pacifico Phototherapy Service, San Gallicano Dermatological Institute, IRCCS, Rome, Italy Abstract. Several topical treatments such as ointments, keratolytics, dithranol, tar, corticosteroids and Vita- min D3 analogues are commonly used in the treatment of mild and/or moderate psoriasis. These treatments can be associated with a variety of local and systemic side effects, as well as to very often unsatisfactory re- sults. The purpose of this critical review of the literature is to evaluate the efficacy and tolerability of the syn- thesis of new analogues of the Vitamin D3 Tacalcitol, which is formulated in ointment form at a concentra- tion of 4 μg/g, for the treatment of mild and/or moderate psoriasis (involvement of <20% of the surface of the skin) and to evaluate whether this drug can be used in the treatment of other skin conditions. Based on existing data in the literature, Tacalcitol is an effective drug for the topical treatment of psoriasis and is also able to ensure that the effects last over time, even after treatment has stopped. Tacalcitol is also well tolerat- ed because the onset of side effects, such as local irritation, pruriginous or burning sensations, were reported in only a small percentage of the subjects who were treated. Lastly, the marked regulatory effects it has on the proliferation and differentiation of keratinocytes, as well as on the immunocompetent cells, has led to suggestions that Tacalcitol may be used in other keratinisation disorders and in some hyperproliferative skin diseases. -

Phototoxicity of 7-Oxycoumarins with Keratinocytes in Culture T ⁎ Christophe Guillona, , Yi-Hua Janb, Diane E
Bioorganic Chemistry 89 (2019) 103014 Contents lists available at ScienceDirect Bioorganic Chemistry journal homepage: www.elsevier.com/locate/bioorg Phototoxicity of 7-oxycoumarins with keratinocytes in culture T ⁎ Christophe Guillona, , Yi-Hua Janb, Diane E. Heckc, Thomas M. Marianob, Robert D. Rappa, Michele Jettera, Keith Kardosa, Marilyn Whittemored, Eric Akyeaa, Ivan Jabine, Jeffrey D. Laskinb, Ned D. Heindela a Department of Chemistry, Lehigh University, Bethlehem, PA 18015, USA b Department of Environmental and Occupational Health, Rutgers University School of Public Health, Piscataway, NJ 08854, USA c Department of Environmental Science, New York Medical College, Valhalla, NY 10595, USA d Buckman Laboratories, 1256 N. McLean Blvd, Memphis, TN 38108, USA e Laboratoire de Chimie Organique, Université Libre de Bruxelles, B-1050 Brussels, Belgium ARTICLE INFO ABSTRACT Keywords: Seventy-one 7-oxycoumarins, 66 synthesized and 5 commercially sourced, were tested for their ability to inhibit 7-hydroxycoumarins growth in murine PAM212 keratinocytes. Forty-nine compounds from the library demonstrated light-induced 7-oxycoumarins lethality. None was toxic in the absence of UVA light. Structure-activity correlations indicate that the ability of Furocoumarins the compounds to inhibit cell growth was dependent not only on their physiochemical characteristics, but also Psoralens on their ability to absorb UVA light. Relative lipophilicity was an important factor as was electron density in the Methoxsalen pyrone ring. Coumarins with electron withdrawing moieties – cyano and fluoro at C – were considerably less 8-MOP 3 Phototoxicity active while those with bromines or iodine at that location displayed enhanced activity. Coumarins that were PAM212 keratinocytes found to inhibit keratinocyte growth were also tested for photo-induced DNA plasmid nicking. -

Reseptregisteret 2013–2017 the Norwegian Prescription Database
LEGEMIDDELSTATISTIKK 2018:2 Reseptregisteret 2013–2017 Tema: Legemidler og eldre The Norwegian Prescription Database 2013–2017 Topic: Drug use in the elderly Reseptregisteret 2013–2017 Tema: Legemidler og eldre The Norwegian Prescription Database 2013–2017 Topic: Drug use in the elderly Christian Berg Hege Salvesen Blix Olaug Fenne Kari Furu Vidar Hjellvik Kari Jansdotter Husabø Irene Litleskare Marit Rønning Solveig Sakshaug Randi Selmer Anne-Johanne Søgaard Sissel Torheim Utgitt av Folkehelseinstituttet/Published by Norwegian Institute of Public Health Område for Helsedata og digitalisering Avdeling for Legemiddelstatistikk Juni 2018 Tittel/Title: Legemiddelstatistikk 2018:2 Reseptregisteret 2013–2017 / The Norwegian Prescription Database 2013–2017 Forfattere/Authors: Christian Berg, redaktør/editor Hege Salvesen Blix Olaug Fenne Kari Furu Vidar Hjellvik Kari Jansdotter Husabø Irene Litleskare Marit Rønning Solveig Sakshaug Randi Selmer Anne-Johanne Søgaard Sissel Torheim Acknowledgement: Julie D. W. Johansen (English text) Bestilling/Order: Rapporten kan lastes ned som pdf på Folkehelseinstituttets nettsider: www.fhi.no The report can be downloaded from www.fhi.no Grafisk design omslag: Fete Typer Ombrekking: Houston911 Kontaktinformasjon/Contact information: Folkehelseinstituttet/Norwegian Institute of Public Health Postboks 222 Skøyen N-0213 Oslo Tel: +47 21 07 70 00 ISSN: 1890-9647 ISBN: 978-82-8082-926-9 Sitering/Citation: Berg, C (red), Reseptregisteret 2013–2017 [The Norwegian Prescription Database 2013–2017] Legemiddelstatistikk 2018:2, Oslo, Norge: Folkehelseinstituttet, 2018. Tidligere utgaver / Previous editions: 2008: Reseptregisteret 2004–2007 / The Norwegian Prescription Database 2004–2007 2009: Legemiddelstatistikk 2009:2: Reseptregisteret 2004–2008 / The Norwegian Prescription Database 2004–2008 2010: Legemiddelstatistikk 2010:2: Reseptregisteret 2005–2009. Tema: Vanedannende legemidler / The Norwegian Prescription Database 2005–2009. -

Topically Used Herbal Products for the Treatment of Psoriasis – Mechanism of Action, Drug Delivery, Clinical Studies
Reviews 1447 Topically Used Herbal Products for the Treatment of Psoriasis – Mechanism of Action, Drug Delivery, Clinical Studies Authors Anna Herman1, Andrzej P. Herman 2 Affiliations 1 Faculty of Cosmetology, The Academy of Cosmetics and Health Care, Warsaw, Poland 2 Laboratory of Molecular Biology, The Kielanowski Institute of Animal Physiology and Nutrition, Polish Academy of Sciences, Jabłonna, Poland Key words Abstract several electronic databases and literature refer- l" Psoriasis ! ences were used to summarize the current l" herbal products Psoriasis is a chronic inflammatory skin disease knowledge acquired on the basis of animal stud- l" keratinocyte hyper- characterized histologically by hyperproliferation ies and clinical trials regarding herbal products proliferation and aberrant differentiation of epidermal kerati- used to treat psoriasis topically. This review dis- l" inflammatory reaction l" skin barrier nocytes. A wide range of conventional medical cusses the mechanisms of herbal products activ- l" herbal drug delivery systems therapies to treat psoriasis is established, from ities through (1) inhibition of the keratinocyte hy- topical therapies and systemic medications perproliferation and inducing apoptosis, (2) inhi- through to phototherapy or combinations of bition of immune-inflammatory reaction, (3) those. However, most of these therapies have a suppression of phosphorylase kinase (PhK) activ- limited efficacy and may cause a number of side ity, and (4) inhibition of the hedgehog (Hh) sig- effects, including cutaneous atrophy, organ toxic- naling pathway. Moreover, the penetration of ity, carcinogenicity, and broadband immunosup- herbal products through the psoriatic skin barrier, pression, which are restricting their long-term novel herbal drug delivery systems in psoriasis use. Therefore, it would be desirable to use herbal treatment, and possible adverse effects of herbal products as an alternative treatment for psoriasis therapy are discussed. -

Part IV: Basic Considerations of the Psoralens
CORE Metadata, citation and similar papers at core.ac.uk Provided by Elsevier - Publisher Connector THE CHEMISTRY OF THE PSORALENS* W. L. FOWLKS, Ph.D. The psoralens belong to a group of compoundsfled since biological activity of the psoralens and which have been considered as derivatives ofangelicins has been demonstrated. They appear coumarin, the furocoumarins. There are twelveto have specific biochemical properties which different ways a furan ring can be condensed withmay contribute to the survival of certain plant the coumarin molecule and each of the resultingspecies. Specifically these compounds belong to compounds could be the parent for a family ofthat group of substances which can inhibit certain derivatives. Examples of most of these possibleplant growth without otherwise harming the furocoumarins have been synthesized; but natureplant (2, 3, 5). is more conservative so that all of the naturally It is interesting that it was this property which occurring furocoumarins so far described turnled to the isolation of the only new naturally out to be derivativesof psoralen I or angelicinoccuring furocoumarin discovered in the United II (1). States. Bennett and Bonner (2) isolated tharn- nosmin from leaves of the Desert Rue (Tham- n.osma montana) because a crude extract of this 0 0 plant was the best growth inhibitor found among /O\/8/O\/ the extracts of a number of desert plants sur- i' Ii veyed for this property, although all the extracts showed seedling growth inhibition. One could I II speculate as to the role such growth inhibition plays in the economy of those desert plants These natural derivatives of psoralen and angeli-when survival may depend upon a successful cm have one or more of the following substituentsfight for the little available water. -

CMP 2 Psoriasis with Acitretin/Methotrexate
1CMP Psoriasis with Acitretin Name of Patient: Patient medication sensitivities/allergies: Patient identification e.g. ID number, date of birth: Current medication: Medical history: Independent Prescriber(s): Supplementary prescriber(s): Dr Sam Gibbs Jill Peters Dermatology Nurse Practitioner Contact details: 01473 704042 Contact details: 01473 704386 or 0787056578 [email protected] [email protected] Condition(s) to be treated: Aim of treatment: Psoriasis – scalp, flexural, guttate, small and large To achieve temporary clearance of the psoriasis or a maintainable plaque and palmer planter psoriasis level of clearance for the patient Medicines that my be prescribed by SP: Preparation Indication Dose schedule Specific indications for referral (All listed preparation may be back to the IP used as sole or conjunct therapy) Mild to potent topical Guttate or small plaque, As detailed in BNF Acute exacerbation or Failure to steroids palmo plantar psoriasis. Finger tip units respond to treatment Cautious use on face and flexural areas (moderate) Facial, scalp or flexural, palmo plantar psoriasis Coal Tar preparations Guttate or small plaque including scalp Dithranol preparations Moderate to large plaque As detailed in BNF as short stable psoriasis contact therapy Salicylic acid 2-10% in WSP Hyperkeratotic psoriasis on scalp or palmo plantar Vitamin D analogue Second line therapy to Use for 4 weeks and then change combined with potent topical moderate/large plaque to Vitamin D alone steroid psoriasis if unresponsive -
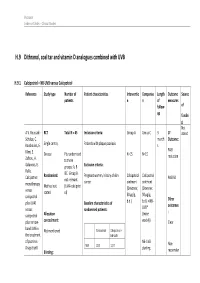
H.9 Dithranol, Coal Tar and Vitamin D Analogues Combined with UVB
Psoriasis Evidence Tables – Clinical Studies H.9 Dithranol, coal tar and vitamin D analogues combined with UVB H.9.1 Calcipotriol + NB-UVB versus Calcipotriol Reference Study type Number of Patient characteristics Interventio Compariso Length Outcome Source patients n n of measures follow- of up fundin g Not A.V. Roussaki- RCT Total N = 45 Inclusion criteria: Group A Group C 3 1º stated Schulze, C. month Outcome: Kouskoukis, E. Single centre, Patients with plaque psoriasis s Klimi, E. PASI Greece Pts randomised N=15 N=15 reduction Zafirou, A. to three Galanous, E. groups: A, B Exclusion criteria: Rallis. &C. Group B Calcipotriol Randomised: Pregnant women, history of skin Calcipotriol Calcipotriol PASI 50 not relevant cancer ointment ointment monotherapy Method not (UVA+calcipotri versus (Dovonex; (Dovonex stated ol) 50 µg/g, 50 µg/g, calcipotriol Other plus UVA1 Baseline characteristics of b.d.) b.d.) + NB- UVB* outcomes versus randomised patients: : calcipotriol Allocation (twice plus narrow- concealment: weekly) Clear band UVB in Calcipotriol Calcipotriol + Not mentioned the treatment NB-UVB of psoriasis. NB-UVB Non- Drugs Exptl. M/F 12/3 12/3 starting Blinding: responder Psoriasis Evidence Tables – Clinical Studies Cl in. Res , dose 80% 31(5/6):169- Not mentioned Age 44.93±6.48 49.53±22.01 MED and 174.2005 inc. by 20% Skin type 0/11/3/1 2/5/6/2 I/II/III/IV every 3 REFID: Washout sessions ROUSSAKISCH period: ULZE2005 90 days if using systemic *Cosmetico therapy, 30 days , 10 lamps if using topicals Helarium B1, 100 W each. -

Daivonex, Topical Ointment
NEW ZEALAND DATA SHEET 1 DAIVONEX® 50 microgram/gram topical ointment 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Daivonex® ointment contains calcipotriol 50 microgram per gram Daivonex® ointment contains the anhydrous form of calcipotriol. For full list of excipients, see section 6.1 List of excipients. 3 PHARMACEUTICAL FORM Daivonex® is a topical ointment. It is a smooth, white preservative free ointment base. 4 CLINICAL PARTICULARS 4.1 Indications Daivonex® ointment is indicated for the topical treatment of psoriasis vulgaris, including plaque psoriasis in adults and children (see section 4.4 Special warnings and precautions for use - paediatric population). In adult patients, Daivonex® ointment may also be used in combination with systemic acitretin or cyclosporin. 4.2 Dosage and method of administration Adults Daivonex® ointment therapy: Daivonex® ointment should be applied topically to the affected area once or twice daily (i.e. in the morning and/or in the evening). Initially, twice daily application of the ointment is usually preferred. Application may then be reduced to once daily, provided individual clinical response is satisfactory. After satisfactory improvement has occurred, treatment should be discontinued. If recurrence develops after reduction in frequency of application or after discontinuation, the treatment may be reinstituted at the initial dosage. Experience is lacking in the use of calcipotriol for periods longer than 1 year. The maximum recommended weekly dose of Daivonex® ointment is 100 g/week. When using a combination of ointment and cream the total maximum dose should not exceed 100 g per week. eDoc-000783454 - Version 1. 0 It should be noted that there are no long-term clinical studies assessing the safety of using Daivonex® ointment during exposure to sunlight. -

Jp Xvii the Japanese Pharmacopoeia
JP XVII THE JAPANESE PHARMACOPOEIA SEVENTEENTH EDITION Official from April 1, 2016 English Version THE MINISTRY OF HEALTH, LABOUR AND WELFARE Notice: This English Version of the Japanese Pharmacopoeia is published for the convenience of users unfamiliar with the Japanese language. When and if any discrepancy arises between the Japanese original and its English translation, the former is authentic. The Ministry of Health, Labour and Welfare Ministerial Notification No. 64 Pursuant to Paragraph 1, Article 41 of the Law on Securing Quality, Efficacy and Safety of Products including Pharmaceuticals and Medical Devices (Law No. 145, 1960), the Japanese Pharmacopoeia (Ministerial Notification No. 65, 2011), which has been established as follows*, shall be applied on April 1, 2016. However, in the case of drugs which are listed in the Pharmacopoeia (hereinafter referred to as ``previ- ous Pharmacopoeia'') [limited to those listed in the Japanese Pharmacopoeia whose standards are changed in accordance with this notification (hereinafter referred to as ``new Pharmacopoeia'')] and have been approved as of April 1, 2016 as prescribed under Paragraph 1, Article 14 of the same law [including drugs the Minister of Health, Labour and Welfare specifies (the Ministry of Health and Welfare Ministerial Notification No. 104, 1994) as of March 31, 2016 as those exempted from marketing approval pursuant to Paragraph 1, Article 14 of the Same Law (hereinafter referred to as ``drugs exempted from approval'')], the Name and Standards established in the previous Pharmacopoeia (limited to part of the Name and Standards for the drugs concerned) may be accepted to conform to the Name and Standards established in the new Pharmacopoeia before and on September 30, 2017. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole