Daivonex, Topical Ointment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vitamin B12ointment Containing Avocado Oil in the Therapy of Plaque
A Service of Leibniz-Informationszentrum econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible. zbw for Economics Stücker, Markus; Memmel, Ulrike; Hoffmann, Matthias; Hartung, Joachim; Altmeyer, Peter Working Paper Vitamin B12 ointment containing avocado oil in the therapy of plaque psoriasis Technical Report, No. 2001,27 Provided in Cooperation with: Collaborative Research Center 'Reduction of Complexity in Multivariate Data Structures' (SFB 475), University of Dortmund Suggested Citation: Stücker, Markus; Memmel, Ulrike; Hoffmann, Matthias; Hartung, Joachim; Altmeyer, Peter (2001) : Vitamin B12 ointment containing avocado oil in the therapy of plaque psoriasis, Technical Report, No. 2001,27, Universität Dortmund, Sonderforschungsbereich 475 - Komplexitätsreduktion in Multivariaten Datenstrukturen, Dortmund This Version is available at: http://hdl.handle.net/10419/77100 Standard-Nutzungsbedingungen: Terms of use: Die Dokumente auf EconStor dürfen zu eigenen wissenschaftlichen Documents in EconStor may be saved and copied for your Zwecken und zum Privatgebrauch gespeichert und kopiert werden. personal and scholarly purposes. Sie dürfen die Dokumente nicht für öffentliche oder kommerzielle You are not to copy documents for public or commercial Zwecke vervielfältigen, öffentlich ausstellen, öffentlich zugänglich purposes, to exhibit the documents publicly, to make them machen, vertreiben oder anderweitig nutzen. publicly available on the internet, or to distribute or otherwise use the documents in public. Sofern die Verfasser die Dokumente unter Open-Content-Lizenzen (insbesondere CC-Lizenzen) zur Verfügung gestellt haben sollten, If the documents have been made available under an Open gelten abweichend von diesen Nutzungsbedingungen die in der dort Content Licence (especially Creative Commons Licences), you genannten Lizenz gewährten Nutzungsrechte. may exercise further usage rights as specified in the indicated licence. -

A Left/Right Comparison of Twice-Daily Calcipotriol Ointment and Calcitriol Ointment in Patients with Psoriasis: the Effect on Keratinocyte Subpopulations
Acta Derm Venereol 2004; 84: 195–200 INVESTIGATIVE REPORT A Left/Right Comparison of Twice-Daily Calcipotriol Ointment and Calcitriol Ointment in Patients with Psoriasis: The Effect on Keratinocyte Subpopulations Mannon E.J. FRANSSEN, Gys J. DE JONGH, Piet E.J. VAN ERP and Peter C.M. VAN DE KERKHOF Department of Dermatology, University Medical Centre Nijmegen, The Netherlands Vitamin D3 analogues are a first-line treatment of Calcipotriol (Daivonex1,50mg/g ointment, Leo chronic plaque psoriasis, but so far, comparative clinical Pharmaceutical Products, Denmark) has been investi- studies on calcipotriol and calcitriol ointment are sparse, gated intensively during the last decade, and has proven and in particular no comparative studies are available on to be a valuable tool in the management of chronic cell biological effects of these compounds in vivo. Using plaque psoriasis. A review by Ashcroft et al. (1), based on flow cytometric assessment, we investigated whether these a large number of randomized controlled trials, showed compounds had different effects on the composition and that calcipotriol was at least as effective as potent DNA synthesis of epidermal cell populations responsible topical corticosteroids, 1a,-25-dihydroxycholecalciferol for the psoriatic phenotype. For 8 weeks, 20 patients with (calcitriol), short-contact dithranol, tacalcitol and coal psoriasis vulgaris were treated twice daily with calcipo- tar. Recently, Scott et al. (2) presented an overview of triol and calcitriol ointment in a left/right comparative studies on the use of calcipotriol ointment in the study. Before and after treatment, clinical assessment of management of psoriasis. They reconfirmed the super- target lesions was performed, together with flow cyto- ior efficacy of a twice-daily calcipotriol ointment metric analysis of epidermal subpopulations with respect regimen to the treatments as mentioned above, and to keratin (K) 10, K6, vimentin and DNA distribution. -

Daily Lifestyle Modifications to Improve Quality of Life And
brain sciences Review Daily Lifestyle Modifications to Improve Quality of Life and Survival in Glioblastoma: A Review Sarah Travers and N. Scott Litofsky * Division of Neurosurgery, University of Missouri School of Medicine, Columbia, MO 65212, USA; [email protected] * Correspondence: [email protected] Abstract: Survival in glioblastoma remains poor despite advancements in standard-of-care treatment. Some patients wish to take a more active role in their cancer treatment by adopting daily lifestyle changes to improve their quality of life or overall survival. We review the available literature through PubMed and Google Scholar to identify laboratory animal studies, human studies, and ongoing clinical trials. We discuss which health habits patients adopt and which have the most promise in glioblastoma. While results of clinical trials available on these topics are limited, dietary restrictions, exercise, use of supplements and cannabis, and smoking cessation all show some benefit in the comprehensive treatment of glioblastoma. Marital status also has an impact on survival. Further clinical trials combining standard treatments with lifestyle modifications are necessary to quantify their survival advantages. Keywords: survival in glioblastoma; dietary restriction in glioblastoma; cannabis use in glioblastoma; supplementation in glioblastoma; glioblastoma health modifications Citation: Travers, S.; Litofsky, N.S. Daily Lifestyle Modifications to 1. Introduction Improve Quality of Life and Survival Glioblastoma remains the most aggressive and deadly form of primary brain tumor, in Glioblastoma: A Review. Brain Sci. with average survival rates ranging from 7.8 to 23.4 months after diagnosis [1]. Maximal 2021, 11, 533. https://doi.org/ surgical resection followed by radiation and temozolomide have become standard of 10.3390/brainsci11050533 care [2,3]. -

Pre-Treatment Vitamin B12, Folate, Ferritin, and Vitamin D Serum Levels
28 RESEARCH ARTICLE Croat Med J. 2020;61:28-32 https://doi.org/10.3325/cmj.2020.61.28 Pre-treatment vitamin B12, Funda Tamer1, Mehmet Eren Yuksel2, Yavuz folate, ferritin, and vitamin D Karabag3 1Department of Dermatology, Gazi serum levels in patients with University School of Medicine, warts: a retrospective study Ankara, Turkey 2Department of General Surgery, Aksaray University School of Medicine, Aksaray, Turkey 3Department of Cardiology, Kafkas University School of Medicine, Kars, Turkey Aim To compare the serum levels of 25-hydroxyvitamin D, ferritin, folate, vitamin B12, zinc, and thyroid stimulating hormone between patients with warts and healthy indi- viduals. Methods This retrospective study enrolled 40 patients with warts and 40 healthy individuals treated at the Ufuk University Hospital, Ankara, between July and December 2017. Serum levels of 25-hydroxyvitamin D, ferritin, folate, vitamin B12, zinc, and thyroid stimulating hormone status were evaluated retrospectively. Results Participants with and without warts had simi- lar mean serum 25-hydroxyvitamin D, ferritin, folate, zinc, and thyroid stimulating hormone levels. However, patients with warts had significantly lower mean serum vitamin B12 level (P = 0.010). Patients with warts non-significantly more frequently had decreased serum levels of 25-hydroxyvita- min D, ferritin, and folate (P = 0.330, P = 0.200, P = 0.070, re- spectively). Conclusion Patients with warts may require evaluation of serum levels of vitamin B12, folate, ferritin, and vitamin D. Received: September 7, 2019 Accepted: January 13, 2020 Correspondence to: Funda Tamer Gazi Universitesi Tıp Fakultesi Mevlana Bulvari, No: 29 06560 Ankara, Turkey [email protected] www.cmj.hr Tamer et al: Vitamin B12, folate, ferritin, and vitamin D in patients with warts 29 Warts are benign epithelial proliferations caused by hu- tion. -

Vitamin D and Immunomodulation in the Skin: a Useful Affirmative Nexus Saptadip Samanta*
Exploration of Immunology Open Access Review Vitamin D and immunomodulation in the skin: a useful affirmative nexus Saptadip Samanta* Department of Physiology, Midnapore College, Midnapore, Paschim Medinipur, West Bengal 721101, India *Correspondence: Saptadip Samanta, Department of Physiology, Midnapore College, Midnapore, Paschim Medinipur, West Bengal 721101, India. [email protected] Academic Editor: Masutaka Furue, Kyushu University, Japan Received: April 2, 2021 Accepted: June 2, 2021 Published: June 30, 2021 Cite this article: Samanta S. Vitamin D and immunomodulation in the skin: a useful affirmative nexus. Explor Immunol. 2021;1:90-111. https://doi.org/10.37349/ei.2021.00009 Abstract Skin is the largest organ of the body having multifunctional activities. It has a dynamic cellular network with unique immunologic properties to maintain defensive actions, photoprotection, immune response, inflammation, tolerogenic capacity, wound healing, etc. The immune cells of the skin exhibit distinct properties. They can synthesize active vitamin D [1,24(OH)2D3] and express vitamin D receptors. Any difficulties in the cutaneous immune system cause skin diseases (psoriasis, vitiligo, atopic dermatitis, skin carcinoma, and others). Vitamin D is an essential factor, exhibits immunomodulatory effects by regulating dendritic cells’ maturation, lymphocytes’ functions, and cytokine production. More specifically, vitamin D acts as an immune balancing agent, inhibits the exaggeration of immunostimulation. This vitamin suppresses T-helper 1 and T-helper 17 cell formation decreases inflammatory cytokines release and promotes the maturation of regulatory T cells and interleukin 10 secretion. The deficiency of this vitamin promotes the occurrence of immunoreactive disorders. Administration of vitamin D or its analogs is the therapeutic choice for the treatment of several skin diseases. -

Reseptregisteret 2013–2017 the Norwegian Prescription Database
LEGEMIDDELSTATISTIKK 2018:2 Reseptregisteret 2013–2017 Tema: Legemidler og eldre The Norwegian Prescription Database 2013–2017 Topic: Drug use in the elderly Reseptregisteret 2013–2017 Tema: Legemidler og eldre The Norwegian Prescription Database 2013–2017 Topic: Drug use in the elderly Christian Berg Hege Salvesen Blix Olaug Fenne Kari Furu Vidar Hjellvik Kari Jansdotter Husabø Irene Litleskare Marit Rønning Solveig Sakshaug Randi Selmer Anne-Johanne Søgaard Sissel Torheim Utgitt av Folkehelseinstituttet/Published by Norwegian Institute of Public Health Område for Helsedata og digitalisering Avdeling for Legemiddelstatistikk Juni 2018 Tittel/Title: Legemiddelstatistikk 2018:2 Reseptregisteret 2013–2017 / The Norwegian Prescription Database 2013–2017 Forfattere/Authors: Christian Berg, redaktør/editor Hege Salvesen Blix Olaug Fenne Kari Furu Vidar Hjellvik Kari Jansdotter Husabø Irene Litleskare Marit Rønning Solveig Sakshaug Randi Selmer Anne-Johanne Søgaard Sissel Torheim Acknowledgement: Julie D. W. Johansen (English text) Bestilling/Order: Rapporten kan lastes ned som pdf på Folkehelseinstituttets nettsider: www.fhi.no The report can be downloaded from www.fhi.no Grafisk design omslag: Fete Typer Ombrekking: Houston911 Kontaktinformasjon/Contact information: Folkehelseinstituttet/Norwegian Institute of Public Health Postboks 222 Skøyen N-0213 Oslo Tel: +47 21 07 70 00 ISSN: 1890-9647 ISBN: 978-82-8082-926-9 Sitering/Citation: Berg, C (red), Reseptregisteret 2013–2017 [The Norwegian Prescription Database 2013–2017] Legemiddelstatistikk 2018:2, Oslo, Norge: Folkehelseinstituttet, 2018. Tidligere utgaver / Previous editions: 2008: Reseptregisteret 2004–2007 / The Norwegian Prescription Database 2004–2007 2009: Legemiddelstatistikk 2009:2: Reseptregisteret 2004–2008 / The Norwegian Prescription Database 2004–2008 2010: Legemiddelstatistikk 2010:2: Reseptregisteret 2005–2009. Tema: Vanedannende legemidler / The Norwegian Prescription Database 2005–2009. -

Topically Used Herbal Products for the Treatment of Psoriasis – Mechanism of Action, Drug Delivery, Clinical Studies
Reviews 1447 Topically Used Herbal Products for the Treatment of Psoriasis – Mechanism of Action, Drug Delivery, Clinical Studies Authors Anna Herman1, Andrzej P. Herman 2 Affiliations 1 Faculty of Cosmetology, The Academy of Cosmetics and Health Care, Warsaw, Poland 2 Laboratory of Molecular Biology, The Kielanowski Institute of Animal Physiology and Nutrition, Polish Academy of Sciences, Jabłonna, Poland Key words Abstract several electronic databases and literature refer- l" Psoriasis ! ences were used to summarize the current l" herbal products Psoriasis is a chronic inflammatory skin disease knowledge acquired on the basis of animal stud- l" keratinocyte hyper- characterized histologically by hyperproliferation ies and clinical trials regarding herbal products proliferation and aberrant differentiation of epidermal kerati- used to treat psoriasis topically. This review dis- l" inflammatory reaction l" skin barrier nocytes. A wide range of conventional medical cusses the mechanisms of herbal products activ- l" herbal drug delivery systems therapies to treat psoriasis is established, from ities through (1) inhibition of the keratinocyte hy- topical therapies and systemic medications perproliferation and inducing apoptosis, (2) inhi- through to phototherapy or combinations of bition of immune-inflammatory reaction, (3) those. However, most of these therapies have a suppression of phosphorylase kinase (PhK) activ- limited efficacy and may cause a number of side ity, and (4) inhibition of the hedgehog (Hh) sig- effects, including cutaneous atrophy, organ toxic- naling pathway. Moreover, the penetration of ity, carcinogenicity, and broadband immunosup- herbal products through the psoriatic skin barrier, pression, which are restricting their long-term novel herbal drug delivery systems in psoriasis use. Therefore, it would be desirable to use herbal treatment, and possible adverse effects of herbal products as an alternative treatment for psoriasis therapy are discussed. -

Calcipotriol/Betamethasone (Dovobet®) in Psoriasis
Bulletin 90 | March 2015 Community Interest Company Calcipotriol/betamethasone (Dovobet®) in psoriasis This bulletin focuses on calcipotriol/betamethasone (Dovobet®) for the treatment of psoriasis. The annual spend on Dovobet® across England is £28.7 million (ePACT October - December 2014). QIPP projects in this area are aimed at reviewing the appropriate use and duration of treatment of Dovobet®. A 50% reduction in prescribing through stopping prolonged treatment could result in potential annual savings of £9 million across England (ePACT October - December 2014). Support materials (briefing, aide-memoire, data collection and patient letter) are available at: http://www.prescqipp.info/resources/viewcategory/326-dovobet-in-psoriasis Recommendations • Vitamin D analogue monotherapy (calcipotriol, calcitriol, tacalcitol) or corticosteroid therapy are first line treatments for the majority of patients with chronic plaque psoriasis.1 Calcitriol ointment is the least expensive vitamin D analogue based on cost per gram. Calcipotriol ointment is the least expensive vitamin D analogue preparation if calculated using the maximum daily application over a four week period (see tables 1 and 2). • Dovobet® is a vitamin D analogue and steroid combination product which should only be considered for poor responders.1 • Dovobet® is recommended as a 4th line treatment for trunk and limb psoriasis in adults but not children or adolescents.1 • Dovobet® gel is recommended as a 3rd line treatment for scalp psoriasis in adults and children or adolescents. Use in children is unlicensed and initiation should be restricted to secondary care.1 • Dovobet® should not be used for the face, flexures or genitals in adults or children.1 • Dovobet® should only be used once daily and for a maximum duration of 4 weeks1 and should only be prescribed as acute treatment. -

Nurse-Led Drug Monitoring Clinic Protocol for the Use of Systemic Therapies in Dermatology for Patients
Group arrangements: Salford Royal NHS Foundation Trust (SRFT) Pennine Acute Hospitals NHS Trust (PAT) Nurse-led drug monitoring clinic protocol for the use of systemic therapies in dermatology for patients with inflammatory dermatoses Lead Author: Dawn Lavery Dermatology Advanced Nurse Practitioner Additional author(s) N/A Division/ Department:: Dermatology, Clinical Support and Tertiary Medicine Applies to: (Please delete) Salford Royal Care Organisation Approving Committee Dermatology clinical governance committee Salford Royal Date approved: 13 February 2019 Expiry date: February 2022 Contents Contents Section Page Document summary sheet 1 Overview 2 2 Scope & Associated Documents 2 3 Background 3 4 What is new in this version? 3 5 Policy 4 Drugs monitored by nurses 4 Acitretin 7 Alitretinoin Toctino 11 Apremilast 22 Azathioprine 26 Ciclosporin 29 Dapsone 34 Fumaric Acid Esters – Fumaderm and Skilarence 36 Hydroxycarbamide 39 Hydroxychloroquine 43 Methotrexate 50 Mycophenolate moefetil 57 Nurse-led drug monitoring clinic protocol for the use of systemic therapies in dermatology for patients with inflammatory dermatoses Reference Number GSCDerm01(13) Version 3 Issue Date: 11/06/2019 Page 1 of 77 It is your responsibility to check on the intranet that this printed copy is the latest version Standards 67 6 Roles and responsibilities 67 7 Monitoring document effectiveness 67 8 Abbreviations and definitions 68 9 References 68 10 Appendices N/A 11 Document Control Information 71 12 Equality Impact Assessment (EqIA) screening tool 73 Group arrangements: Salford Royal NHS Foundation Trust (SRFT) Pennine Acute Hospitals NHS Trust (PAT) 1. Overview (What is this policy about?) The dermatology directorate specialist nurses are responsible for ensuring prescribing and monitoring for patients under their care, is in accordance with this protocol. -

CMP 2 Psoriasis with Acitretin/Methotrexate
1CMP Psoriasis with Acitretin Name of Patient: Patient medication sensitivities/allergies: Patient identification e.g. ID number, date of birth: Current medication: Medical history: Independent Prescriber(s): Supplementary prescriber(s): Dr Sam Gibbs Jill Peters Dermatology Nurse Practitioner Contact details: 01473 704042 Contact details: 01473 704386 or 0787056578 [email protected] [email protected] Condition(s) to be treated: Aim of treatment: Psoriasis – scalp, flexural, guttate, small and large To achieve temporary clearance of the psoriasis or a maintainable plaque and palmer planter psoriasis level of clearance for the patient Medicines that my be prescribed by SP: Preparation Indication Dose schedule Specific indications for referral (All listed preparation may be back to the IP used as sole or conjunct therapy) Mild to potent topical Guttate or small plaque, As detailed in BNF Acute exacerbation or Failure to steroids palmo plantar psoriasis. Finger tip units respond to treatment Cautious use on face and flexural areas (moderate) Facial, scalp or flexural, palmo plantar psoriasis Coal Tar preparations Guttate or small plaque including scalp Dithranol preparations Moderate to large plaque As detailed in BNF as short stable psoriasis contact therapy Salicylic acid 2-10% in WSP Hyperkeratotic psoriasis on scalp or palmo plantar Vitamin D analogue Second line therapy to Use for 4 weeks and then change combined with potent topical moderate/large plaque to Vitamin D alone steroid psoriasis if unresponsive -

Vitamin D and Its Analogues Decrease Amyloid- (A) Formation
International Journal of Molecular Sciences Article Vitamin D and Its Analogues Decrease Amyloid-β (Aβ) Formation and Increase Aβ-Degradation Marcus O. W. Grimm 1,2,3,*,† ID , Andrea Thiel 1,† ID , Anna A. Lauer 1 ID , Jakob Winkler 1, Johannes Lehmann 1,4, Liesa Regner 1, Christopher Nelke 1, Daniel Janitschke 1,Céline Benoist 1, Olga Streidenberger 1, Hannah Stötzel 1, Kristina Endres 5, Christian Herr 6 ID , Christoph Beisswenger 6, Heike S. Grimm 1 ID , Robert Bals 6, Frank Lammert 4 and Tobias Hartmann 1,2,3 1 Experimental Neurology, Saarland University, Kirrberger Str. 1, 66421 Homburg/Saar, Germany; [email protected] (A.T.); [email protected] (A.A.L.); [email protected] (J.W.); [email protected] (J.L.); [email protected] (L.R.); [email protected] (C.N.); [email protected] (D.J.); [email protected] (C.B.); [email protected] (O.S.); [email protected] (H.S.); [email protected] (H.S.G.); [email protected] (T.H.) 2 Neurodegeneration and Neurobiology, Saarland University, Kirrberger Str. 1, 66421 Homburg/Saar, Germany 3 Deutsches Institut für DemenzPrävention (DIDP), Saarland University, Kirrberger Str. 1, 66421 Homburg/Saar, Germany 4 Department of Internal Medicine II–Gastroenterology, Saarland University Hospital, Saarland University, Kirrberger Str. 100, 66421 Homburg/Saar, Germany; [email protected] 5 Department of Psychiatry and Psychotherapy, Clinical Research Group, University Medical Centre Johannes Gutenberg, University of Mainz, Untere Zahlbacher Str. 8, 55131 Mainz, Germany; [email protected] 6 Department of Internal Medicine V–Pulmonology, Allergology, Respiratory Intensive Care Medicine, Saarland University Hospital, Kirrberger Str. -
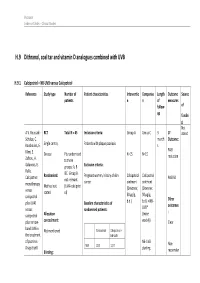
H.9 Dithranol, Coal Tar and Vitamin D Analogues Combined with UVB
Psoriasis Evidence Tables – Clinical Studies H.9 Dithranol, coal tar and vitamin D analogues combined with UVB H.9.1 Calcipotriol + NB-UVB versus Calcipotriol Reference Study type Number of Patient characteristics Interventio Compariso Length Outcome Source patients n n of measures follow- of up fundin g Not A.V. Roussaki- RCT Total N = 45 Inclusion criteria: Group A Group C 3 1º stated Schulze, C. month Outcome: Kouskoukis, E. Single centre, Patients with plaque psoriasis s Klimi, E. PASI Greece Pts randomised N=15 N=15 reduction Zafirou, A. to three Galanous, E. groups: A, B Exclusion criteria: Rallis. &C. Group B Calcipotriol Randomised: Pregnant women, history of skin Calcipotriol Calcipotriol PASI 50 not relevant cancer ointment ointment monotherapy Method not (UVA+calcipotri versus (Dovonex; (Dovonex stated ol) 50 µg/g, 50 µg/g, calcipotriol Other plus UVA1 Baseline characteristics of b.d.) b.d.) + NB- UVB* outcomes versus randomised patients: : calcipotriol Allocation (twice plus narrow- concealment: weekly) Clear band UVB in Calcipotriol Calcipotriol + Not mentioned the treatment NB-UVB of psoriasis. NB-UVB Non- Drugs Exptl. M/F 12/3 12/3 starting Blinding: responder Psoriasis Evidence Tables – Clinical Studies Cl in. Res , dose 80% 31(5/6):169- Not mentioned Age 44.93±6.48 49.53±22.01 MED and 174.2005 inc. by 20% Skin type 0/11/3/1 2/5/6/2 I/II/III/IV every 3 REFID: Washout sessions ROUSSAKISCH period: ULZE2005 90 days if using systemic *Cosmetico therapy, 30 days , 10 lamps if using topicals Helarium B1, 100 W each.