Draft Guidance on Tazarotene
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Left/Right Comparison of Twice-Daily Calcipotriol Ointment and Calcitriol Ointment in Patients with Psoriasis: the Effect on Keratinocyte Subpopulations
Acta Derm Venereol 2004; 84: 195–200 INVESTIGATIVE REPORT A Left/Right Comparison of Twice-Daily Calcipotriol Ointment and Calcitriol Ointment in Patients with Psoriasis: The Effect on Keratinocyte Subpopulations Mannon E.J. FRANSSEN, Gys J. DE JONGH, Piet E.J. VAN ERP and Peter C.M. VAN DE KERKHOF Department of Dermatology, University Medical Centre Nijmegen, The Netherlands Vitamin D3 analogues are a first-line treatment of Calcipotriol (Daivonex1,50mg/g ointment, Leo chronic plaque psoriasis, but so far, comparative clinical Pharmaceutical Products, Denmark) has been investi- studies on calcipotriol and calcitriol ointment are sparse, gated intensively during the last decade, and has proven and in particular no comparative studies are available on to be a valuable tool in the management of chronic cell biological effects of these compounds in vivo. Using plaque psoriasis. A review by Ashcroft et al. (1), based on flow cytometric assessment, we investigated whether these a large number of randomized controlled trials, showed compounds had different effects on the composition and that calcipotriol was at least as effective as potent DNA synthesis of epidermal cell populations responsible topical corticosteroids, 1a,-25-dihydroxycholecalciferol for the psoriatic phenotype. For 8 weeks, 20 patients with (calcitriol), short-contact dithranol, tacalcitol and coal psoriasis vulgaris were treated twice daily with calcipo- tar. Recently, Scott et al. (2) presented an overview of triol and calcitriol ointment in a left/right comparative studies on the use of calcipotriol ointment in the study. Before and after treatment, clinical assessment of management of psoriasis. They reconfirmed the super- target lesions was performed, together with flow cyto- ior efficacy of a twice-daily calcipotriol ointment metric analysis of epidermal subpopulations with respect regimen to the treatments as mentioned above, and to keratin (K) 10, K6, vimentin and DNA distribution. -

Profile of Clinical Efficacy and Safety of Topical Tacalcitol
leone 9-06-2005 14:07 Pagina 13 ACTA BIO MED 2005; 76; 13-19 © Mattioli 1885 U PDATE Profile of clinical efficacy and safety of topical tacalcitol Giovanni Leone, Alessia Pacifico Phototherapy Service, San Gallicano Dermatological Institute, IRCCS, Rome, Italy Abstract. Several topical treatments such as ointments, keratolytics, dithranol, tar, corticosteroids and Vita- min D3 analogues are commonly used in the treatment of mild and/or moderate psoriasis. These treatments can be associated with a variety of local and systemic side effects, as well as to very often unsatisfactory re- sults. The purpose of this critical review of the literature is to evaluate the efficacy and tolerability of the syn- thesis of new analogues of the Vitamin D3 Tacalcitol, which is formulated in ointment form at a concentra- tion of 4 μg/g, for the treatment of mild and/or moderate psoriasis (involvement of <20% of the surface of the skin) and to evaluate whether this drug can be used in the treatment of other skin conditions. Based on existing data in the literature, Tacalcitol is an effective drug for the topical treatment of psoriasis and is also able to ensure that the effects last over time, even after treatment has stopped. Tacalcitol is also well tolerat- ed because the onset of side effects, such as local irritation, pruriginous or burning sensations, were reported in only a small percentage of the subjects who were treated. Lastly, the marked regulatory effects it has on the proliferation and differentiation of keratinocytes, as well as on the immunocompetent cells, has led to suggestions that Tacalcitol may be used in other keratinisation disorders and in some hyperproliferative skin diseases. -

An Empirical Study on Laboratory Coke Oven-Based Coal Blending for Coking with Tar Residue, Chemical Engineering Transactions, 71, 385-390 DOI:10.3303/CET1871065 386
385 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 71, 2018 The Italian Association of Chemical Engineering Online at www.aidic.it/cet Guest Editors: Xiantang Zhang, Songrong Qian, Jianmin Xu Copyright © 2018, AIDIC Servizi S.r.l. ISBN 978-88-95608-68-6; ISSN 2283-9216 DOI: 10.3303/CET1871065 An Empirical Study on Laboratory Coke Oven-based Coal Blending for Coking with Tar Residue Wenqiu Liu Hebei Energy College of Vocation and Technology, Tangshan 063004, China [email protected] Tar residue is the solid waste produced in coking plants or gas generators. Utilizing tar residue as additive in coal blending for coking is an effective approach for coal coking in our country, not only improving the yield and nature of coking products, but also realizing the recycling of tar residue. In this paper, tar residue is used as additive in coal blending for coking experiments, and combined with experiments on the crucible coke, small coke oven and industrial coke oven, the influences of the addition amount of tar residue on coke yield, coke reactivity and coke strength after reaction are further analyzed. As the experiment results reveal, compared to the experiment results of crucible coke and small coke ovens, the coke yield, coke reactivity and coke strength after reaction of industrial coke ovens are all bigger, leading to potential industrialization of coal blending for coking. Moreover, the influence of the amount of tar residue on the coal tar yield rate and gas yield is the same as that of coke reactivity and coke strength after reaction. 1. Introduction With the rapid development of economy, the fast growth of iron and steel industry has led China's coke industry to accomplish extraordinary achievements (Si et al., 2017). -

Coal Characteristics
CCTR Indiana Center for Coal Technology Research COAL CHARACTERISTICS CCTR Basic Facts File # 8 Brian H. Bowen, Marty W. Irwin The Energy Center at Discovery Park Purdue University CCTR, Potter Center, 500 Central Drive West Lafayette, IN 47907-2022 http://www.purdue.edu/dp/energy/CCTR/ Email: [email protected] October 2008 1 Indiana Center for Coal Technology Research CCTR COAL FORMATION As geological processes apply pressure to peat over time, it is transformed successively into different types of coal Source: Kentucky Geological Survey http://images.google.com/imgres?imgurl=http://www.uky.edu/KGS/coal/images/peatcoal.gif&imgrefurl=http://www.uky.edu/KGS/coal/coalform.htm&h=354&w=579&sz= 20&hl=en&start=5&um=1&tbnid=NavOy9_5HD07pM:&tbnh=82&tbnw=134&prev=/images%3Fq%3Dcoal%2Bphotos%26svnum%3D10%26um%3D1%26hl%3Den%26sa%3DX 2 Indiana Center for Coal Technology Research CCTR COAL ANALYSIS Elemental analysis of coal gives empirical formulas such as: C137H97O9NS for Bituminous Coal C240H90O4NS for high-grade Anthracite Coal is divided into 4 ranks: (1) Anthracite (2) Bituminous (3) Sub-bituminous (4) Lignite Source: http://cc.msnscache.com/cache.aspx?q=4929705428518&lang=en-US&mkt=en-US&FORM=CVRE8 3 Indiana Center for Coal Technology Research CCTR BITUMINOUS COAL Bituminous Coal: Great pressure results in the creation of bituminous, or “soft” coal. This is the type most commonly used for electric power generation in the U.S. It has a higher heating value than either lignite or sub-bituminous, but less than that of anthracite. Bituminous coal -

Coal Tar Pitch – Its Past, Present and Future in Commercial Roofing by Joe Mellott
- 1 - Coal Tar Pitch – Its Past, Present and Future in Commercial Roofing By Joe Mellott Coal tar remains a desired and strong source of technology within the roofing industry, as innovative coal tar products significantly reduce associated health hazards and environmental impact. To help you better understand the role that coal tar continues to play in the commercial roofing market, this article will explore: • The history of coal tar • Its associated hazards • Hot and cold coal tar adhesive technologies • Modified coal tar pitch membrane technologies • Coal tar’s sustainability attributes A Brief History of Coal Tar In order to understand what coal tar pitch is, it’s important to understand its origins and refinement methods. Coal tar can be refined from a number of sources including coal, wood, peat, petroleum, and other organic materials. The tar is removed by burning or heating the base substance and selectively distilling fractions of the burned chemical. Distillation involves heating the substance to a point where different fractions of the substance become volatile. The fractions are then collected by condensing the fraction at a specific temperature. A base substance can be split into any number of fractions through distillation. A good example of industrial distillation is the oil refining process. Through distillation, crude oil can be separated into fractions that include gasoline, jet fuel, motor oil bases, and other specialty chemicals. Fractionation or distillation is a tried-and-true method for breaking a substance into different parts of its composition. One of the first uses of coal tar was in the maritime industry. Trees stumps were burned and the tar fractions were collected through distillation of the tar. -

Spherical Graphite Produced by Waste Semi-Coke with Enhanced Properties As
Electronic Supplementary Material (ESI) for Sustainable Energy & Fuels. This journal is © The Royal Society of Chemistry 2019 Spherical graphite produced by waste semi-coke with enhanced properties as anode material for Li-ion batteries Ming Shi, Zige Tai, Na Li, Kunyang Zou, Yuanzhen Chen, Junjie Sun, Yongning Liu* State Key Laboratory for Mechanical Behavior of Materials, School of Material Science and Engineering, Xi’an Jiaotong University, Xi’an 710049, PR China *Corresponding author E-mail address: [email protected] Fax: +86 29 8266 3453; Tel: +86 29 8266 4602 Fig. 1 N2 absorption/desorption profiles of (a) pristine semi-coke (SC); (b) synthetic graphite without Si (PG); and (c) synthetic graphite with 10% Si at 2300 °C (SG). Table 1 The percentage of impurity of pristine SC and SG (10% Si at 2300 °C). Content percentage / wt % samples B Si Al Ca Fe K Na Mg SC <0.1 4.25 5.5 3.3 0.8 0.3 0.09 <0.1 SG <0.1 1.01 1.9 0.1 <0.1 0.02 0.03 <0.1 Table 2 The SG capacity values with that of similar materials published in the literature. Materials Specific Capacity Rate capability Cyclic retention Ref. [mA h g-1] [mA h g-1] Spherical graphite produced 347.06 at 0.05C 329.8 at 0.1C; 317.4 at 97.7% at 0.5C after This by waste semi-coke 0.5 C and 262.3 at 1C 700 cycles work Iron‐catalyzed graphitic 306 at 0.1C 150 at rate of 2C Over 90 % after 200 1 materials from biomass cycles Carbonaceous composites 312 at 0.2C 149 at 1 C; 78 at 5C - 2 prepared by the mixture of graphite, cokes, and petroleum pitch. -

Lecture 32: Coke Production
NPTEL – Chemical – Chemical Technology II Lecture 32: Coke production 32.1 Introduction Coal is used as fuel for electric power generation, industrial heating and steam generation, domestic heating, rail roads and for coal processing. Coal composition is denoted by rank. Rank increases with the carbon content and decreases with increasing oxygen content. Many of the products made by hydrogenation, oxidation, hydrolysis or fluorination are important for industrial use. Stable, low cost, petroleum and natural gas supplies has arose interest in some of the coal products as upgraded fuels to reduce air pollution as well as to take advantage of greater ease of handling of the liquid or gaseous material and to utilize existing facilities such as pipelines and furnaces. 32.2 Coking of coal Raw material is Bituminous coal. It appears to have specific internal surfaces in the range of 30 to 100m2/g. Generally one ton of bituminous coal produces 1400 lb of coke. 10 gallons of tar. Chemical reaction:- 4(C3H4)n nC6H6 + 5nC + 3nH2 + nCH4 Coal Benzene Coke Lighter hydrocarbon Joint initiative of IITs and IISc – Funded by MHRD Page 1 of 9 NPTEL – Chemical – Chemical Technology II Process flow sheet: Illustrated in Figure. Figure 32.1 Flow sheet of coking of coal 32.3 Functional role of each unit (Figure 32.1): (a) Coal crusher and screening: At first Bituminous coal is crushed and screened to a certain size. Preheating of coal (at 150-250˚C) is done to reduce coking time without loss of coal quality. Briquetting increases strength of coke produced and to make non - coking or poorly coking coals to be used as metallurgical coke. -
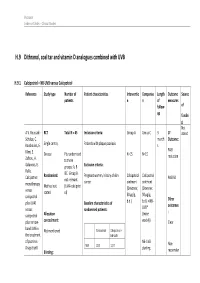
H.9 Dithranol, Coal Tar and Vitamin D Analogues Combined with UVB
Psoriasis Evidence Tables – Clinical Studies H.9 Dithranol, coal tar and vitamin D analogues combined with UVB H.9.1 Calcipotriol + NB-UVB versus Calcipotriol Reference Study type Number of Patient characteristics Interventio Compariso Length Outcome Source patients n n of measures follow- of up fundin g Not A.V. Roussaki- RCT Total N = 45 Inclusion criteria: Group A Group C 3 1º stated Schulze, C. month Outcome: Kouskoukis, E. Single centre, Patients with plaque psoriasis s Klimi, E. PASI Greece Pts randomised N=15 N=15 reduction Zafirou, A. to three Galanous, E. groups: A, B Exclusion criteria: Rallis. &C. Group B Calcipotriol Randomised: Pregnant women, history of skin Calcipotriol Calcipotriol PASI 50 not relevant cancer ointment ointment monotherapy Method not (UVA+calcipotri versus (Dovonex; (Dovonex stated ol) 50 µg/g, 50 µg/g, calcipotriol Other plus UVA1 Baseline characteristics of b.d.) b.d.) + NB- UVB* outcomes versus randomised patients: : calcipotriol Allocation (twice plus narrow- concealment: weekly) Clear band UVB in Calcipotriol Calcipotriol + Not mentioned the treatment NB-UVB of psoriasis. NB-UVB Non- Drugs Exptl. M/F 12/3 12/3 starting Blinding: responder Psoriasis Evidence Tables – Clinical Studies Cl in. Res , dose 80% 31(5/6):169- Not mentioned Age 44.93±6.48 49.53±22.01 MED and 174.2005 inc. by 20% Skin type 0/11/3/1 2/5/6/2 I/II/III/IV every 3 REFID: Washout sessions ROUSSAKISCH period: ULZE2005 90 days if using systemic *Cosmetico therapy, 30 days , 10 lamps if using topicals Helarium B1, 100 W each. -

Projectprofile
EPSILON CARBON PRIVATE LIMITED ECPL CoalTar Distillation -300,000tpa Karnataka ProjectProfile General TheEpsilon CarbonprivateLimited is aspiring to emergeasadynamiccompanyin India in the field of coal tar processing for manufacture ofvarious value added products. The proposed green-field Coal tar distillation plant coupledwith solidpitchmeltingunitisproposedtosetupwith Coal tar processing capacity of 300,000tpaat Toranagallu in Bellary district of Karnataka. Process Description: The coal tar are separated by distillation process. Coal tar containing several chemical compounds with major being Coal tar pitch,Naphthalene,Wash Oil, Light Oil, Phenol Oil & Anthracene oil. Following are major manufacturing of process & Products: 1. Coal Tar pitch (Binder Grade Pitch) 2. Zero QI /Impregnated Pitch 3. Naphthalene &Naphthalene sulfonate formaldehyde (NSF) 4. Oils- Carbon Black Oil (CBO) ,Light oil, Phenol oil, Wash oil , Anthracene oil By Products: 5. Sodium Phenolate 6. Ammonical water 1. Binder Grade Pitch: Soft Pitch from the bottom of pitch column is sent to pitch column for further processing to modified pitch to increase QI content and blended with required high QI pitch to produce binder grade pitch for aluminum industry. 2. Impregnated Pitch : Blending of coal tar and wash oil in specific ratio, dehydration up to 180℃ to separate moisture content in coal tar. The dehydrated mixture is transferred to settling tank for reduction of QI. (Settling tank provide draw-off valve for QI sampling). QI value less material transfer to further distillation (Removal of Oils) to produce Impregnated Pitch. Project Profile for 300,000 tpa Coal Tar Distillation plant EPSILON CARBON PRIVATE LIMITED ECPL CoalTar Distillation -300,000tpa Karnataka ProjectProfile 3. Carbon Black Oil(CBO): Blending of Soft Pitch from the bottom of pitch column and Anthracene oil in specific ratio with required specific gravity to produce carbon black oil for tyre industry. -

Interpreting Tar Patterns at Former Manufactured Gas Plant Sites
1 Interpreting Tar Patterns at Former Manufactured Gas Plant Sites Brian L. Murphy, Ph.D. • INEF, Penn State • June 10, 2013 2 Why focus on tar? . Dense, nonaqueous-phase liquid (DNAPL) that can sink into the saturated zone and contaminate groundwater – Expensive to remediate . Insurance coverage litigation – How tar got there? (Expected and intended?) – When tar got there? (During coverage period?) – Is it still moving? (During coverage period?) 3 Outline . What is manufactured gas plant (MGP) tar? . How “tarry” is it? . How fast does it move? – Distance and time scales . How to interpret boring logs in terms of tar motion . How can you identify source locations at a site where tar has migrated? 4 Nomenclature . “Tar” generally = Density > Water . “Oil” generally = Density < Water . “Coal tar” = Tar from different processes – Coal gasification – Water gas – Carbureted water gas – Oil gas Which may not involve the use of coal at all! 5 Different Processes for Manufacturing Gas . Coal gas: Heat coal in the absence of oxygen . Water gas (blue gas): Steam sprayed on incandescent coke H2O+C→CO+H2 . Carbureted water gas: Petroleum is cracked and added to water gas—most popular Lowe process . Oil gas: Cracked petroleum for low molecular weight gases 6 Initial Coal Gas Period . 1812: First commercial plant chartered, London . First U.S. gas companies incorporated – 1817 Baltimore – 1823 New York – 1829 Boston 7 Advent of Water Gas . 1873: Lowe Carbureted Water Gas (CWG) Process patented; water gas provided superior illumination – 1884: Patents sold to United Gas Improvement Company – 1892: Lowe’s patents begin to expire . 1900: CWG accounts for 75% of U.S. -

Coal-Modified Tar Binders for Bituminous Concrete Pavements
Coal-Modified Tar Binders for Bituminous Concrete Pavements EDMUND O. RHODES, Curtiss-Wright Corporation, Pittsburgh, Pennsylvania In Fall 1958, investigations were started by the Re• search Division of Curtiss-Wright Corp. at Quehanna, Pa., with a view to developing improved methods for using bituminous coals and products derived there• from as highway construction materials. It was decided to explore the possibility of making an improved binder for bituminous pavements of the hot-mix hot-lay type by dispersing coal in distilled coal tars and coal tar oils. Previous investigations had indicated that they might also be used to advantage for the production of improved highway binders. During the first half of 1959 a task force at Que• hanna assembled and constructed suitable laboratory and pilot plant equipment; determined optimum condi• tions for the dispersion of coal m tars and oils; com• pared various coals, tars, and oils as to their suita• bility for the purpose; produced and analyzed experi• mental quantities of coal-modified tar binders; and combined them with various aggregates in hot mixes. The latter were then compared with hot mixes con• taining typical asphalt cements and coal-tar binders. The tests appeared to indicate that it would be possible to make hot mixes with coal-modified tar cements equal or superior to those made with usual asphalt or coal-tar binders. After the results of the Quehanna investigations were reported, a contract was made with the Com• monwealth of Kentucky to build a pilot plant at Frank• fort to produce 150,000 gal of coal-modified tar binder for comparison with asphalt cements normally used in Kentucky in Class I and Class I-modified bitumi• nous pavements. -

Jp Xvii the Japanese Pharmacopoeia
JP XVII THE JAPANESE PHARMACOPOEIA SEVENTEENTH EDITION Official from April 1, 2016 English Version THE MINISTRY OF HEALTH, LABOUR AND WELFARE Notice: This English Version of the Japanese Pharmacopoeia is published for the convenience of users unfamiliar with the Japanese language. When and if any discrepancy arises between the Japanese original and its English translation, the former is authentic. The Ministry of Health, Labour and Welfare Ministerial Notification No. 64 Pursuant to Paragraph 1, Article 41 of the Law on Securing Quality, Efficacy and Safety of Products including Pharmaceuticals and Medical Devices (Law No. 145, 1960), the Japanese Pharmacopoeia (Ministerial Notification No. 65, 2011), which has been established as follows*, shall be applied on April 1, 2016. However, in the case of drugs which are listed in the Pharmacopoeia (hereinafter referred to as ``previ- ous Pharmacopoeia'') [limited to those listed in the Japanese Pharmacopoeia whose standards are changed in accordance with this notification (hereinafter referred to as ``new Pharmacopoeia'')] and have been approved as of April 1, 2016 as prescribed under Paragraph 1, Article 14 of the same law [including drugs the Minister of Health, Labour and Welfare specifies (the Ministry of Health and Welfare Ministerial Notification No. 104, 1994) as of March 31, 2016 as those exempted from marketing approval pursuant to Paragraph 1, Article 14 of the Same Law (hereinafter referred to as ``drugs exempted from approval'')], the Name and Standards established in the previous Pharmacopoeia (limited to part of the Name and Standards for the drugs concerned) may be accepted to conform to the Name and Standards established in the new Pharmacopoeia before and on September 30, 2017.