Spatial Identification of Tributary Impacts in River Networks
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(P 117-140) Flood Pulse.Qxp
117 THE FLOOD PULSE CONCEPT: NEW ASPECTS, APPROACHES AND APPLICATIONS - AN UPDATE Junk W.J. Wantzen K.M. Max-Planck-Institute for Limnology, Working Group Tropical Ecology, P.O. Box 165, 24302 Plön, Germany E-mail: [email protected] ABSTRACT The flood pulse concept (FPC), published in 1989, was based on the scientific experience of the authors and published data worldwide. Since then, knowledge on floodplains has increased considerably, creating a large database for testing the predictions of the concept. The FPC has proved to be an integrative approach for studying highly diverse and complex ecological processes in river-floodplain systems; however, the concept has been modified, extended and restricted by several authors. Major advances have been achieved through detailed studies on the effects of hydrology and hydrochemistry, climate, paleoclimate, biogeography, biodi- versity and landscape ecology and also through wetland restoration and sustainable management of flood- plains in different latitudes and continents. Discussions on floodplain ecology and management are greatly influenced by data obtained on flow pulses and connectivity, the Riverine Productivity Model and the Multiple Use Concept. This paper summarizes the predictions of the FPC, evaluates their value in the light of recent data and new concepts and discusses further developments in floodplain theory. 118 The flood pulse concept: New aspects, INTRODUCTION plain, where production and degradation of organic matter also takes place. Rivers and floodplain wetlands are among the most threatened ecosystems. For example, 77 percent These characteristics are reflected for lakes in of the water discharge of the 139 largest river systems the “Seentypenlehre” (Lake typology), elaborated by in North America and Europe is affected by fragmen- Thienemann and Naumann between 1915 and 1935 tation of the river channels by dams and river regula- (e.g. -

Calvin Park Tributary Stream Restoration -- Survey Phase
Neighborhood Advisory Croydon Creek – Calvin Park Tributary Stream Restoration -- Survey Phase Project Rockville’s Department of Public Works has begun the design of the Croydon Creek – Description: Calvin Park Tributary Stream Restoration project. The city’s consultant, AECOM, will survey the area within Rockville Civic Center Park in the project limits depicted on the back side of this advisory. The survey will identify features including the existing roads, stream, trees, utilities, and topography. Survey work will begin in March and will continue into the spring. Surveyors and engineers with equipment will traverse the area shown on the back of this advisory. Survey stakes may be placed in the ground and trees may be marked with temporary metal tags to assist in identification and inventory. The survey flags do not indicate tree removal. Purpose: The Croydon Creek – Calvin Park Tributary Stream Restoration project was recommended in the 2013 Rock Creek Watershed Assessment as a crucial component to the long-term health of the watershed. The assessment was approved by the Mayor and Council in February 2013, and this project was included in the adopted Fiscal Year 2017 Capital Improvements Program. The project goals are to enhance the watershed through stream restoration, wetland enhancement, reforestation, and the protection of private and public property and infrastructure Location: Croydon Creek within Rockville Civic Center Park and the Calvin Park Tributary from Baltimore Road to Croydon Creek (See the map on the back of this advisory). Timeline: Survey work and preliminary design is anticipated to begin in March and continue through spring. A community meeting will be held this summer to provide additional details of the project. -

Geomorphic Classification of Rivers
9.36 Geomorphic Classification of Rivers JM Buffington, U.S. Forest Service, Boise, ID, USA DR Montgomery, University of Washington, Seattle, WA, USA Published by Elsevier Inc. 9.36.1 Introduction 730 9.36.2 Purpose of Classification 730 9.36.3 Types of Channel Classification 731 9.36.3.1 Stream Order 731 9.36.3.2 Process Domains 732 9.36.3.3 Channel Pattern 732 9.36.3.4 Channel–Floodplain Interactions 735 9.36.3.5 Bed Material and Mobility 737 9.36.3.6 Channel Units 739 9.36.3.7 Hierarchical Classifications 739 9.36.3.8 Statistical Classifications 745 9.36.4 Use and Compatibility of Channel Classifications 745 9.36.5 The Rise and Fall of Classifications: Why Are Some Channel Classifications More Used Than Others? 747 9.36.6 Future Needs and Directions 753 9.36.6.1 Standardization and Sample Size 753 9.36.6.2 Remote Sensing 754 9.36.7 Conclusion 755 Acknowledgements 756 References 756 Appendix 762 9.36.1 Introduction 9.36.2 Purpose of Classification Over the last several decades, environmental legislation and a A basic tenet in geomorphology is that ‘form implies process.’As growing awareness of historical human disturbance to rivers such, numerous geomorphic classifications have been de- worldwide (Schumm, 1977; Collins et al., 2003; Surian and veloped for landscapes (Davis, 1899), hillslopes (Varnes, 1958), Rinaldi, 2003; Nilsson et al., 2005; Chin, 2006; Walter and and rivers (Section 9.36.3). The form–process paradigm is a Merritts, 2008) have fostered unprecedented collaboration potentially powerful tool for conducting quantitative geo- among scientists, land managers, and stakeholders to better morphic investigations. -

The Arkansas River Flood of June 3-5, 1921
DEPARTMENT OF THE INTERIOR ALBERT B. FALL, Secretary UNITED STATES GEOLOGICAL SURVEY GEORGE 0ns SMITH, Director Water-Supply Paper 4$7 THE ARKANSAS RIVER FLOOD OF JUNE 3-5, 1921 BY ROBERT FOLLANS^EE AND EDWARD E. JON^S WASHINGTON GOVERNMENT PRINTING OFFICE 1922 i> CONTENTS. .Page. Introduction________________ ___ 5 Acknowledgments ___ __________ 6 Summary of flood losses-__________ _ 6 Progress of flood crest through Arkansas Valley _____________ 8 Topography of Arkansas basin_______________ _________ 9 Cause of flood______________1___________ ______ 11 Principal areas of intense rainfall____ ___ _ 15 Effect of reservoirs on the flood__________________________ 16 Flood flows_______________________________________ 19 Method of determination________________ ______ _ 19 The flood between Canon City and Pueblo_________________ 23 The flood at Pueblo________________________________ 23 General features_____________________________ 23 Arrival of tributary flood crests _______________ 25 Maximum discharge__________________________ 26 Total discharge_____________________________ 27 The flood below Pueblo_____________________________ 30 General features _________ _______________ 30 Tributary streams_____________________________ 31 Fountain Creek____________________________ 31 St. Charles River___________________________ 33 Chico Creek_______________________________ 34 Previous floods i____________________________________ 35 Flood of Indian legend_____________________________ 35 Floods of authentic record__________________________ 36 Maximum discharges -

Lower Wisconsin River Main Stem
LOWER WISCONSIN RIVER MAIN STEM The Wisconsin River begins at Lac Vieux Desert, a lake in Vilas County that lies on the border of Wisconsin and the Lower Wisconsin River Upper Pennisula in Michigan. The river is approximately At A Glance 430 miles long and collects water from 12,280 square miles. As a result of glaciation across the state, the river Drainage Area: 4,940 sq. miles traverses a variety of different geologic and topographic Total Stream Miles: 165 miles settings. The section of the river known as the Lower Wisconsin River crosses over several of these different Major Public Land: geologic settings. From the Castle Rock Flowage, the river ♦ Units of the Lower Wisconsin flows through the flat Central Sand Plain that is thought to State Riverway be a legacy of Glacial Lake Wisconsin. Downstream from ♦ Tower Hill, Rocky Arbor, and Wisconsin Dells, the river flows through glacial drift until Wyalusing State Parks it enters the Driftless Area and eventually flows into the ♦ Wildlife areas and other Mississippi River (Map 1, Chapter Three ). recreation areas adjacent to river Overall, the Lower Wisconsin River portion of the Concerns and Issues: Wisconsin River extends approximately 165 miles from the ♦ Nonpoint source pollution Castle Rock Flowage dam downstream to its confluence ♦ Impoundments with the Mississippi River near Prairie du Chien. There are ♦ Atrazine two major hydropower dams operate on the Lower ♦ Fish consumption advisories Wisconsin, one at Wisconsin Dells and one at Prairie Du for PCB’s and mercury Sac. The Wisconsin Dells dam creates Kilbourn Flowage. ♦ Badger Army Ammunition The dam at Prairie Du Sac creates Lake Wisconsin. -

Classifying Rivers - Three Stages of River Development
Classifying Rivers - Three Stages of River Development River Characteristics - Sediment Transport - River Velocity - Terminology The illustrations below represent the 3 general classifications into which rivers are placed according to specific characteristics. These categories are: Youthful, Mature and Old Age. A Rejuvenated River, one with a gradient that is raised by the earth's movement, can be an old age river that returns to a Youthful State, and which repeats the cycle of stages once again. A brief overview of each stage of river development begins after the images. A list of pertinent vocabulary appears at the bottom of this document. You may wish to consult it so that you will be aware of terminology used in the descriptive text that follows. Characteristics found in the 3 Stages of River Development: L. Immoor 2006 Geoteach.com 1 Youthful River: Perhaps the most dynamic of all rivers is a Youthful River. Rafters seeking an exciting ride will surely gravitate towards a young river for their recreational thrills. Characteristically youthful rivers are found at higher elevations, in mountainous areas, where the slope of the land is steeper. Water that flows over such a landscape will flow very fast. Youthful rivers can be a tributary of a larger and older river, hundreds of miles away and, in fact, they may be close to the headwaters (the beginning) of that larger river. Upon observation of a Youthful River, here is what one might see: 1. The river flowing down a steep gradient (slope). 2. The channel is deeper than it is wide and V-shaped due to downcutting rather than lateral (side-to-side) erosion. -

Drainage Patterns
Drainage Patterns Over time, a stream system achieves a particular drainage pattern to its network of stream channels and tributaries as determined by local geologic factors. Drainage patterns or nets are classified on the basis of their form and texture. Their shape or pattern develops in response to the local topography and Figure 1 Aerial photo illustrating subsurface geology. Drainage channels develop where surface dendritic pattern in Gila County, AZ. runoff is enhanced and earth materials provide the least Courtesy USGS resistance to erosion. The texture is governed by soil infiltration, and the volume of water available in a given period of time to enter the surface. If the soil has only a moderate infiltration capacity and a small amount of precipitation strikes the surface over a given period of time, the water will likely soak in rather than evaporate away. If a large amount of water strikes the surface then more water will evaporate, soaks into the surface, or ponds on level ground. On sloping surfaces this excess water will runoff. Fewer drainage channels will develop where the surface is flat and the soil infiltration is high because the water will soak into the surface. The fewer number of channels, the coarser will be the drainage pattern. Dendritic drainage pattern A dendritic drainage pattern is the most common form and looks like the branching pattern of tree roots. It develops in regions underlain by homogeneous material. That is, the subsurface geology has a similar resistance to weathering so there is no apparent control over the direction the tributaries take. -

Brand New Mississippi Sternwheeler from American Cruise Lines
Brand New Mississippi Sternwheeler from American Cruise Lines Guilford, CT - American Cruise Lines has announced it is expanding to the Mississippi River system with a brand new sternwheeler, already under construction at Chesapeake Shipbuilding in Salisbury, MD. It plans to operate the new riverboat on routes similar to those formerly run by Delta Queen Steamboat Company, which will include the Mississippi, Ohio, Tennessee, and Cumberland Rivers. Cruising the Mississippi River on a sternwheeler is a true all-American experience that American Cruise Lines is pleased to bring back. The new paddlewheeler will recreate the grandeur of past riverboats while possessing the latest safety, environmental and construction technologies. The ship will have the look of a traditional riverboat along with more amenities, a faster speed, and an unmatched level of comfort. Features include six unique lounges, a library, an elegant dining salon, elevator service to all decks, and the exceptionally large staterooms found on all American Cruise Lines ships. With only 140 passengers, each guest will receive personalized service in the intimate and friendly atmosphere for which American Cruise Lines has become known. The first cruise is a scheduled to depart August 11, 2012 from New Orleans, Louisiana on a 7-night journey up the Mississippi to Memphis, Tennessee. The ship will then begin a series of 7-night cruises travelling as far north as St. Paul, Minnesota while utilizing its remarkable speed to open up new itinerary possibilities. As on all true riverboats, a stage and bow ramp will give the ship access to the many interesting ports without docking facilities. -

Watershed Technical Analysis
Trout Creek Watershed Stormwater Management Plan SECTION III: WATERSHED TECHNICAL ANALYSIS A. Watershed Modeling An initial step this study of the Trout Creek watershed was the selection of a stormwater simulation model to be used in the analysis. To provide a reasonable estimate of existing flows within the watershed to serve as a baseline for the study of stormwater management and to effectively evaluate the hydrologic response of the watershed to various changes in stormwater management it was necessary to select a model which: • Modeled design storms of various durations and frequencies to produce routed hydrographs which could be combined • Was adaptable to the size of subwatersheds in this study • Could evaluate specific physical characteristics of the rainfall-runoff process • Did not require an excessive amount of input data yet yielded reliable results The model selected for use in this study was the U. S. Army Corps of Engineers, Hydrologic Engineering Center, Hydrologic Modeling System (HEC-HMS) for the following reasons: • It had been developed at the Hydrologic Engineering Center specifically for the analysis of the timing of surface flow contributions to peak rates at various locations in a watershed • Although originally developed as an urban runoff simulation model, data requirements make it easily adaptable to a rural situation • Input parameters provide a flexible calibration process • It has the ability to analyze reservoir or detention basin routing effects • It is accepted by the Pennsylvania Department of Environmental Protection Although other models, such as TR-20, may provide essentially the same results as the HEC-HMS, HMS’s ability to compare subwatershed contributions in a peak flow presentation table make it specifically attractive for this study. -
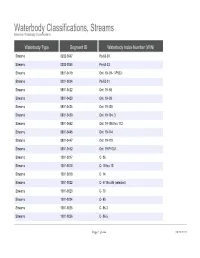
Waterbody Classifications, Streams Based on Waterbody Classifications
Waterbody Classifications, Streams Based on Waterbody Classifications Waterbody Type Segment ID Waterbody Index Number (WIN) Streams 0202-0047 Pa-63-30 Streams 0202-0048 Pa-63-33 Streams 0801-0419 Ont 19- 94- 1-P922- Streams 0201-0034 Pa-53-21 Streams 0801-0422 Ont 19- 98 Streams 0801-0423 Ont 19- 99 Streams 0801-0424 Ont 19-103 Streams 0801-0429 Ont 19-104- 3 Streams 0801-0442 Ont 19-105 thru 112 Streams 0801-0445 Ont 19-114 Streams 0801-0447 Ont 19-119 Streams 0801-0452 Ont 19-P1007- Streams 1001-0017 C- 86 Streams 1001-0018 C- 5 thru 13 Streams 1001-0019 C- 14 Streams 1001-0022 C- 57 thru 95 (selected) Streams 1001-0023 C- 73 Streams 1001-0024 C- 80 Streams 1001-0025 C- 86-3 Streams 1001-0026 C- 86-5 Page 1 of 464 09/28/2021 Waterbody Classifications, Streams Based on Waterbody Classifications Name Description Clear Creek and tribs entire stream and tribs Mud Creek and tribs entire stream and tribs Tribs to Long Lake total length of all tribs to lake Little Valley Creek, Upper, and tribs stream and tribs, above Elkdale Kents Creek and tribs entire stream and tribs Crystal Creek, Upper, and tribs stream and tribs, above Forestport Alder Creek and tribs entire stream and tribs Bear Creek and tribs entire stream and tribs Minor Tribs to Kayuta Lake total length of select tribs to the lake Little Black Creek, Upper, and tribs stream and tribs, above Wheelertown Twin Lakes Stream and tribs entire stream and tribs Tribs to North Lake total length of all tribs to lake Mill Brook and minor tribs entire stream and selected tribs Riley Brook -

Channel Changes and Flood Frequency on the Upper Main Stem of the Nooksack River, Whatcom County, Washington
Western Washington University Western CEDAR WWU Graduate School Collection WWU Graduate and Undergraduate Scholarship Winter 1993 Channel Changes and Flood Frequency on the Upper Main Stem of the Nooksack River, Whatcom County, Washington Roger G. Bertschi Western Washington University, [email protected] Follow this and additional works at: https://cedar.wwu.edu/wwuet Part of the Geology Commons Recommended Citation Bertschi, Roger G., "Channel Changes and Flood Frequency on the Upper Main Stem of the Nooksack River, Whatcom County, Washington" (1993). WWU Graduate School Collection. 717. https://cedar.wwu.edu/wwuet/717 This Masters Thesis is brought to you for free and open access by the WWU Graduate and Undergraduate Scholarship at Western CEDAR. It has been accepted for inclusion in WWU Graduate School Collection by an authorized administrator of Western CEDAR. For more information, please contact [email protected]. CHANNEL CHANGES AND FLOOD FREQUENCY ON THE UPPER MAIN STEM OF THE NOOKSACK RIVER, WHATCOM COUNTY, WASHINGTON by Roger G. Bertschi Accepted in Partial Completion of the Requirements for the Degree Master of Science Advisory Committee MASTER'S THESIS In presenting this thesis in partial fulfillment of the requirements for a master's degree at Western Washington University, I agree that the Library shall make its copies freely available for inspection. I further agree that extensive copying of this thesis is allowable only for scholarly purposes. It is understood, however, that any copying or publication of this thesis for commercial purposes, or for financial gain, shall not be allowed without mv permission. Signature Date MASTER’S THESIS In presenting this thesis in partial fulfillment of the requirements for a master’s degree at Western Washington University, I grant to Western Washington University the non-exclusive royalty-free right to archive, reproduce, distribute, and display the thesis in any and all forms, including electronic format, via any digital library mechanisms maintained by WWU. -

Protecting and Managing Hudson River Streams: Overview, Scales and Definitions
Protecting and managing Hudson River streams: Overview, scales and definitions A comprehensive approach to stream management yields many benefits for a local community and its water resources including improving water quality, reducing and mitigating flooding, protecting wildlife habitat and maintaining and enhancing public access and recreational activities. Although much attention has been placed on the health of the Hudson River in recent years, resulting in a dramatic improvement in water quality in the river, the health of the many streams and tributaries of the Hudson River Basin also play an important role for the water quality and overall health of the watershed. Overview Streams are part of larger systems called a stream or riparian corridors, which often include floodplains and wetlands adjacent to streams. Streams include the water flowing through them and the land beneath them called the stream bed or channel. Other spatial scales to consider are the lands around the stream – including the stream or riparian buffer, adjacent floodplains and wetlands, upland habitat and watershed. Size and Scale of Watersheds • Everyone lives in a watershed. Wherever you are in the world, you are in a watershed. • A watershed supports a web of life that is interconnected, meaning that every plant and animal interacts with many other organisms in the watershed during their life cycle. A typical watershed is a network of smaller rivers or streams called tributaries, which are connected and eventually flow into a larger stream or river. • Watersheds are divided into smaller drainage areas or subwatersheds. For example, in the Hudson Source: Sandusky River Watershed Coalition Valley, the Quassaick Creek Watershed (56 square miles) and Moodna Creek Watershed (180 square miles) are subwatersheds of the larger Hudson Hudson River Watershed River Watershed, which drains about 13,500 The Hudson River flows from its highest point at square miles of land.