Diane Pennica, Ph.D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chart Book Template
Real Chart Page 1 become a problem, since each track can sometimes be released as a separate download. CHART LOG - F However if it is known that a track is being released on 'hard copy' as a AA side, then the tracks will be grouped as one, or as soon as known. Symbol Explanations s j For the above reasons many remixed songs are listed as re-entries, however if the title is Top Ten Hit Number One hit. altered to reflect the remix it will be listed as would a new song by the act. This does not apply ± Indicates that the record probably sold more than 250K. Only used on unsorted charts. to records still in the chart and the sales of the mix would be added to the track in the chart. Unsorted chart hits will have no position, but if they are black in colour than the record made the Real Chart. Green coloured records might not This may push singles back up the chart or keep them around for longer, nevertheless the have made the Real Chart. The same applies to the red coulered hits, these are known to have made the USA charts, so could have been chart is a sales chart and NOT a popularity chart on people’s favourite songs or acts. Due to released in the UK, or imported here. encryption decoding errors some artists/titles may be spelt wrong, I apologise for any inconvenience this may cause. The chart statistics were compiled only from sales of SINGLES each week. Not only that but Date of Entry every single sale no matter where it occurred! Format rules, used by other charts, where unnecessary and therefore ignored, so you will see EP’s that charted and other strange The Charts were produced on a Sunday and the sales were from the previous seven days, with records selling more than other charts. -

The Inner Shrine a Novel of Today
1 THE INNER SHRINE A NOVEL OF TODAY HARPER & BROTHERS NEW YORK AND LONDON M.C.M.I.X Copyright, 1908, 1909, by HARPER & BROTHERS. All rights reserved. Published May, 1909. I Though she had counted the strokes of every hour since midnight, Mrs. Eveleth had no thought of going to bed. When she was not sitting bolt upright, indifferent to comfort, in one of the stiff-backed, gilded chairs, she was limping, with the aid of her cane, up and down the long suite of salons, listening for the sound of wheels. She knew that George and Diane would be surprised to find her waiting up for them, and that they might even be annoyed; but in her state of dread it was impossible to yield to small considerations. She could hardly tell how this presentiment of disaster had taken hold upon her, for the beginning of it must have come as imperceptibly as the first flicker of dusk across the radiance of an afternoon. Looking back, she could almost make herself believe that she had seen its shadow over her early satisfaction in her son's marriage to Diane. Certainly she had felt it there before their honeymoon was over. The four years that had passed since then had been spent—or, at least, she would have said so now—in waiting for the peril to present itself. And yet, had she been called on to explain why she saw it stalking through the darkness of this particular June night, she would have found it difficult to give coherent statement to her fear. -

Karaoke Mietsystem Songlist
Karaoke Mietsystem Songlist Ein Karaokesystem der Firma Showtronic Solutions AG in Zusammenarbeit mit Karafun. Karaoke-Katalog Update vom: 13/10/2020 Singen Sie online auf www.karafun.de Gesamter Katalog TOP 50 Shallow - A Star is Born Take Me Home, Country Roads - John Denver Skandal im Sperrbezirk - Spider Murphy Gang Griechischer Wein - Udo Jürgens Verdammt, Ich Lieb' Dich - Matthias Reim Dancing Queen - ABBA Dance Monkey - Tones and I Breaking Free - High School Musical In The Ghetto - Elvis Presley Angels - Robbie Williams Hulapalu - Andreas Gabalier Someone Like You - Adele 99 Luftballons - Nena Tage wie diese - Die Toten Hosen Ring of Fire - Johnny Cash Lemon Tree - Fool's Garden Ohne Dich (schlaf' ich heut' nacht nicht ein) - You Are the Reason - Calum Scott Perfect - Ed Sheeran Münchener Freiheit Stand by Me - Ben E. King Im Wagen Vor Mir - Henry Valentino And Uschi Let It Go - Idina Menzel Can You Feel The Love Tonight - The Lion King Atemlos durch die Nacht - Helene Fischer Roller - Apache 207 Someone You Loved - Lewis Capaldi I Want It That Way - Backstreet Boys Über Sieben Brücken Musst Du Gehn - Peter Maffay Summer Of '69 - Bryan Adams Cordula grün - Die Draufgänger Tequila - The Champs ...Baby One More Time - Britney Spears All of Me - John Legend Barbie Girl - Aqua Chasing Cars - Snow Patrol My Way - Frank Sinatra Hallelujah - Alexandra Burke Aber Bitte Mit Sahne - Udo Jürgens Bohemian Rhapsody - Queen Wannabe - Spice Girls Schrei nach Liebe - Die Ärzte Can't Help Falling In Love - Elvis Presley Country Roads - Hermes House Band Westerland - Die Ärzte Warum hast du nicht nein gesagt - Roland Kaiser Ich war noch niemals in New York - Ich War Noch Marmor, Stein Und Eisen Bricht - Drafi Deutscher Zombie - The Cranberries Niemals In New York Ich wollte nie erwachsen sein (Nessajas Lied) - Don't Stop Believing - Journey EXPLICIT Kann Texte enthalten, die nicht für Kinder und Jugendliche geeignet sind. -

Lets Just Say It Wasnt Pretty PDF Book
LETS JUST SAY IT WASNT PRETTY PDF, EPUB, EBOOK Diane Keaton | 224 pages | 29 Apr 2014 | Random House USA Inc | 9780812994261 | English | New York, United States Lets Just Say it Wasnt Pretty PDF Book To a ten-year-old girl, that meant President Lincoln was ugly. She is, after all, a woman who enjoyed a famous affair with Warren Beatty during his Adonis-like prime. More Entertainment. A delightful read that covered a lot of territory in a few pages, I will save this one for a reread…of some of the sections, at least. I am along with Diane, a part of a generation that I am proud to be part of. Let's Just Say it Wasn't Pretty. It was like reading someone's diary and almost fell into the category of "gibberish". It was a very odd experience and made me wax nostalgic to hear it again as well as Ms. An obsession? Javascript is not enabled in your browser. So why has a woman with so little vanity devoted such time and effort to pursuing beauty? Before a particularly emotional scene, I took it out, unfolded Warren, and touched his face with my fingers. I wish you'd left off with Then Again, which was actually a touching four-star read that cemented my support for your work. We are experiencing technical difficulties. In Let's Just Say It Wasn't Pretty, she shares the wisdom she's accumulated through the years as a mother, daughter, actress, artist, and international style icon. That stood out with particular intensity during our bittersweet breakup. -

Scanned Image
Index Bubbling A -Z guide to producers UnderSingles 101-150 and publishers 4 FROM 101 LOVE SONG CHAS & DAVE (ROCKNEY KOR AMOR RAMON ARCUSA (LATIN-AMERICAN/SOUTHERN) 57 16) ANNIE GET YOUR GUN ALAN TARNEY IRONDORI 65 ANNIE, I'M NOT YOUR DADDY AUGUST DARNELL (ISLAND) 31 102 LET'S GO TO BED CURE (FICTION FICSIX) BACK ON THE CHAIN GANG CHRIS THOMAS (HYNDE HOUSE OF 17) HITS/CLIVE BANKS/ ATV) 46 103 SHAME AND SCANDAL CLINT EASTWOOD BACK TO LOVE 1152 & GENERAL SAINT (GREENSLEEVES BE PROUD, BE LOUD IRE HEARD) NICK TAUBER (SWEET 'N. SOUR) (12)OINK 3) H3 104 DEVIL OR ANGEL BILLY FURY (POLYDOR BEST YEARS OF OUR LIVES TONY VISCONTI (APRIL/BUSINESS TODD ARTS) 42 POSP 528) BEWARE BOYFRIEND TONY MANSFIELD (WARNER BROS149 105 CHEERS THEN BANANARAMA (LONDON CACHARPAYA 0 93 NANAINANX) 3) CARIOCA MIKE CHAPMAN (REFORMATION) 96 106 I.G.Y. DONALD FAGEN (WARNER BROS CAROLINE (LIVE) STATUS QUO (VALLEY) 25 9299007) CRY BOY CRY TIM FRIESE-GREENE (MAGNET) 20 107 I'M ALRIGHT YOUNG STEVE & THE DESPERATE BUT NOT SERIOUS 1161 DO IT TO THE MUSIC RON DEAN MILLER (RACHEL ILEOSONGII 24 AFTERNOON BOYS (RCA RCA 296) DO YOU REALLY WANT TO HURT ME STEVE LEVINE (VIRGIN) 10 108 PLAY AT YOUR OWN RISK PLANET PATROL DON'T MAKE ME WAIT LEVAN/DE BENEDICTUS (RACHEL (21/POLYDOR POSP 535) GEOSONG)) 84 TIME HEALS 109 LITTLE TOWN CLIFF RICHARD (EMI EMI DON'T PAY THE FERRYMAN RUPERT HINE (RONDOR) 63 5348) DRACULA'S TANGO BARRY BLUE (BLUE MER /VIRGIN/MAGIC 110 STRAIGHT AHEAD NICK STRAKER FROG/HEATH LEVY/COPYRIGHT CONTROL) 76 (FIREBIRD FLAME 33) DREAMIN' 11 81 EASTWORLD 099 111 A.M. -
2 Column Indented
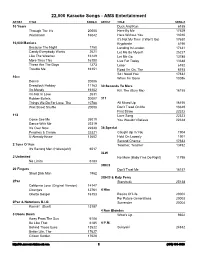
22,000 Karaoke Songs - AMS Entertainment ARTIST TITLE SONG # ARTIST TITLE SONG # 10 Years Duck And Run 6188 Through The Iris 20005 Here By Me 17629 Wasteland 16042 Here Without You 13010 It's Not My Time (I Won't Go) 17630 10,000 Maniacs Kryptonite 6190 Because The Night 1750 Landing In London 17631 Candy Everybody Wants 2621 Let Me Be Myself 25227 Like The Weather 16149 Let Me Go 13785 More Than This 16150 Live For Today 13648 These Are The Days 1273 Loser 6192 Trouble Me 16151 Road I'm On, The 6193 So I Need You 17632 10cc When I'm Gone 13086 Donna 20006 Dreadlock Holiday 11163 30 Seconds To Mars I'm Mandy 16152 Kill, The (Bury Me) 16155 I'm Not In Love 2631 Rubber Bullets 20007 311 Things We Do For Love, The 10788 All Mixed Up 16156 Wall Street Shuffle 20008 Don't Tread On Me 13649 First Straw 22322 112 Love Song 22323 Come See Me 25019 You Wouldn't Believe 22324 Dance With Me 22319 It's Over Now 22320 38 Special Peaches & Cream 22321 Caught Up In You 1904 U Already Know 13602 Hold On Loosely 1901 Second Chance 17633 2 Tons O' Fun Teacher, Teacher 13492 It's Raining Men (Hallelujah!) 6017 3LW 2 Unlimited No More (Baby I'ma Do Right) 11795 No Limits 6183 3Oh!3 20 Fingers Don't Trust Me 16157 Short Dick Man 1962 3OH!3 & Katy Perry 2Pac Starstrukk 25138 California Love (Original Version) 14147 Changes 12761 4 Him Ghetto Gospel 16153 Basics Of Life 20002 For Future Generations 20003 2Pac & Notorious B.I.G. -

Feature Interview
A Lindsey Buckingham Newsletter Special to this Issue – April 21, 2001 What’s New Feature Interview Feature Album Review Feature Performance Review Featured Song Fun & Games ¯ Lindsey Buckingham’s album is still tentatively scheduled for release on Reprise, June 26, 2001. R ¯ VH1’s Behind the Music special is set to air U July 1, 2001. ¯ He may perform some warm up shows in M June in the Los Angeles area. ¯ Mick Fleetwood, Stevie Nicks and Lindsey O Buckingham are planning to start the next Fleetwood Mac album this fall. Mick and R Stevie report that Lindsey has already started working on the songs. S ¯ The Rockline website (http://www.drchuck.net/ rockline/) lists Lindsey & Mick as potential future guests. *~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~*~* Listening Forward In this section, one of Lindsey’s more recent songs to whet the appetite for what’s to come. Steal Your Heart Away (Lindsey Buckingham, 1997) All alone we go on Day after day All alone we suffer Oh, steal your heart away It’s the same old thing In the same old way All alone we suffer So come on Oh, steal your away Let’s go Let’s run away And the light goes creepin’ If that’s all there is Down, down, down Oh, steal your heart away While we were sleepin’ Suddenly we hit the ground The light goes creepin’ Down, down, down While we were sleepin’ Suddenly we hit the ground Oh, steal your heart away So come on (Mmmm, lonely suffer) Let’s go Steal your heart away Let’s run away (Mmmm, lonely suffer) If that’s all there is Steal your heart away Steal your heart away (Mmmm, lonely suffer) Steal your heart away (Mmmm, lonely suffer) Steal your heart away 2 Recording, Engineering and Production August 1992 LINDSEY BUCKINGHAM by Dan Levitin As the arranger/producer behind Fleetwood Mac from 1975 - 1988, Lindsey Buckingham is largely responsible for that group’s superstardom and megasuccess on the five studio albums, Fleetwood Mac, Rumours, Tusk, Mirage and Tango In The Night. -

FLEETWOOD MAC: Die Legendären Klassiker Auf Farbigem Vinyl Fleetwood Mac , Rumours , Tusk , Mirage Und Tango in the Night
im Auftrag: medienAgentur Stefan Michel T 040-5149 1467 F 040-5149 1465 [email protected] FLEETWOOD MAC: Die legendären Klassiker auf farbigem Vinyl Fleetwood Mac , Rumours , Tusk , Mirage und Tango In The Night Ab 29. November über Rhino Limited Editions in individuell nummerierten Slipcases exklusiv über www.Rhino.com FLEETWOOD MAC „Rumours“ 4CD Edition bereits ab 8. November erhältlich! Zwischen 1975 und 1987 setzten FLEETWOOD MAC fünf einzigartige Multi-Platin-Alben in Folge in die Musiklandschaft – ein brillanter Coup, der sie zu einer der weltweit meistverkaufenden Bands in der Rockgeschichte machte! Am 29. November veröffentlicht Rhino diese fünf Klassiker auf Vinyl in jeweils eigener Farbe: Fleetwood Mac auf weißem Vinyl, Rumours auf transparentem Vinyl, Tusk als Doppel-LP auf silberfarbenem Vinyl, Mirage auf violettem Vinyl und Tango In The Night auf grünem Vinyl! Gleichzeitig erscheinen alle fünf Alben gesammelt in einem Slipcase als individuell durchnummerierte, auf 2.000 Exemplare limitierte Edition, die exklusiv über www.Rhino.com zu beziehen sein wird. Diese Sammlung kann ab sofort vorbestellt werden. Zuvor erscheint „Rumours“ am 8. November bereits als 4CD Box. Nach ihren bluesigen Anfängen erschienen FLEETWOOD MAC im Sommer 1975 in einer neuen Inkarnation in neuer Besetzung, die aus Mick Fleetwood, John McVie, Christine McVie und den neuen Mitgliedern Stevie Nicks und Lindsey Buckingham bestand. Ihr erstes gemeinsames Album, Fleetwood Mac (manchmal auch als The White Album bezeichnet), katapultierte sich umgehend auf Platz 1 der Billboard-Charts, hielt sich über ein Jahr in den Top- 40 und verkaufte sich mit Songs wie „Landslide“, „Say You Love Me“ und „Rhiannon“ allein in den USA über fünf Millionen Mal! 1977 warf die Band den Nachfolger Rumours ins Rennen, das vielfach als das beste Album aller Zeiten bewertet wird. -

Karaoke Catalog Updated On: 17/12/2016 Sing Online on in English Karaoke Songs
Karaoke catalog Updated on: 17/12/2016 Sing online on www.karafun.com In English Karaoke Songs (H?D) Planet Earth My One And Only Hawaiian Hula Eyes Blackout I Love My Baby (My Baby Loves Me) On The Beach At Waikiki Other Side I'll Build A Stairway To Paradise Deep In The Heart Of Texas 10 Years My Blue Heaven What Are You Doing New Year's Eve Through The Iris What Can I Say After I Say I'm Sorry Long Ago And Far Away 10,000 Maniacs When You're Smiling (The Whole World Smiles With Bésame mucho (English Vocal) Because The Night 'S Wonderful For Me And My Gal 10CC 1930s Standards 'Til Then Dreadlock Holiday Let's Call The Whole Thing Off Daddy's Little Girl I'm Not In Love Heartaches The Old Lamplighter The Things We Do For Love Cheek To Cheek Someday You'll Want Me To Want You Rubber Bullets Love Is Sweeping The Country That Old Black Magic (Woman Voice) Life Is A Minestrone My Romance That Old Black Magic (Man Voice) 112 It's Time To Say Aloha I Know Why (And So Do You) DUET Cupid We Gather Together Aren't You Glad You're You Peaches And Cream Kumbaya (I've Got A Gal In) Kalamazoo 12 Gauge The Last Time I Saw Paris My One And Only Highland Fling Dunkie Butt All The Things You Are No Love No Nothin' 12 Stones Smoke Gets In Your Eyes Personality Far Away Begin The Beguine Sunday, Monday Or Always Crash I Love A Parade This Heart Of Mine 1800s Standards I Love A Parade (short version) Mister Meadowlark Home Sweet Home I'm Gonna Sit Right Down And Write Myself A Letter 1950s Standards Home On The Range Body And Soul Get Me To The Church On -

Viewed These Women About the Voices They Have Heard, Their Understanding of the Voices, Their Interpersonal Relationships, and Their General Mood and Functioning
MIAMI UNIVERSITY The Graduate School Certificate for Approving the Dissertation We hereby approve the Dissertation of Emily K. Reese Candidate for the Degree: Doctor of Philosophy _________________________________ Chair Larry M. Leitner, Ph.D. _________________________________ Reader Ann Fuehrer, Ph.D. _________________________________ Reader Aaron Luebbe, Ph.D. _________________________________ Graduate School Representative Elise Radina, Ph.D. ABSTRACT VOICES, RELATIONSHIPS, AND MEANING MAKING by Emily K. Reese In this study, I explored the experiences of four women who hear voices and are not part of a clinical population. Each woman had a much different voice hearing experience from the other women in terms of duration, regularity, consistency, and sensory modality. I interviewed these women about the voices they have heard, their understanding of the voices, their interpersonal relationships, and their general mood and functioning. A robust meaning making system and healthy interpersonal relationships were associated with lower distress about the voices, and with lower distress in daily life in general. In addition, connection with a validational community, and to the integral universe (Leitner, 2010), also were associated with lower distress. I present several implications for clinical practice, elaboration of Experiential Personal Construct Psychotherapy theory, and future research. In particular, I challenge the notion that hearing voices is an automatic indicator of “mental illness.” Instead, I argue that relationships and meaning making are far better indicators of psychological health. VOICES, RELATIONSHIPS, AND MEANING MAKING A Dissertation Submitted to the Faculty of Miami University in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Psychology by Emily K. Reese Miami University Oxford, Ohio 2014 Dissertation Chair: Dr. -

Ukeglos Songbook 2 Update 1 City of New Orleans (Steve Goodman, Arlo Guthrie)
Contents Blue Moon Oh Diane / Diana City Of New Orleans Oh Susanna Common People The Old Bazaar In Cairo Cruising Down The River Rave On / Oh Boy / Peggy Sue Dedicated Follower Of Fashion Sailing Folsom Prison Blues Singing The Blues Good As Gold (Stupid As Mud) So Happy Together If I Had A Hammer Summer Nights (Mens' Copy) Iko Iko Summer Nights (Womens' Copy) Its In His Kiss (The Shoop Shoop Suspicious Minds Song) (Mens' Copy) Swing Low Sweet Chariot / When The Its In His Kiss (The Shoop Shoop Saints / This Train Is Bound For Glory Song) (Womens' Copy) Tequila Sunrise Jolene Time Warp Knockin' On Heaven's Door Under The Boardwalk Leaning On A Lamp Post Walk Right Back Leaving On A Jet Plane The Water is Wide (in C) Little Bitty Tear Whiskey In The Jar Maxwell's Silver Hammer Will You Love Me Tomorrow Meet Me On The Corner Yellow Submarine Messing About On The River You've Got To Hide Your Love Away Octopus's Garden Songbook 2 first issued: 2 July 2012 Revision (Update 1) issued: 1 September 2013 Revision (Update 2) issued: 7 September 2014 Blue Moon (Rogers and Hart) Cmaj7 Am7 Intro: Cmaj7 Am7 Dm7 G7 Cmaj7 Am7 Dm7 1. Blue moon, G7 Cmaj7 Am7 Dm7 You saw me standing alone Dm7 G7 G7 Cmaj7 Am7 Dm7 Without a dream in my heart, G7 Cmaj7 Am7 Dm7 G7 Without a love of my own Cmaj7 Am7 Dm7 F Fm 2. Blue moon, G7 Cmaj7 Am7 Dm7 You knew just what I was there for G7 Cmaj7 Am7 Dm7 You heard me saying a prayer for, G7 Cmaj7 F Fm C Dm Am Someone I really could care for Dm G7 C Am Bridge: And then there suddenly appeared before me Dm G7 C The only one my arms will ever hold C Fm 7 Fm7 Bb Eb I heard somebody whisper, "Please adore me" G7 D7 Dm G7 And when I looked the moon had turned to gold Cmaj7 Am7 Dm7 Bb Eb 3. -

PETTY, Tom, and the Heartbreakers 1980S: #25 / 1990S: #25 / All-Time: #79 Born on 10/20/1950 in Gainesville, Florida
G DEBUT PEAK WKS O ARTIST L DATE POS CHR D Album Title Label & Number PETTY, Tom, And The Heartbreakers 1980s: #25 / 1990s: #25 / All-Time: #79 Born on 10/20/1950 in Gainesville, Florida. Died of a heart attack on 10/2/2017 (age 66). Rock singer/songwriter/guitarist. Formed The Heartbreakers in Los Angeles, California: Mike Campbell (guitar; born on 2/1/1950), Benmont Tench (keyboards; born on 9/7/1953), Ron Blair (bass; born on 9/16/1948) and Stan Lynch (drums; born on 5/21/1955). Howie Epstein (born on 7/21/1955; died of a drug overdose on 2/23/2003, age 47) replaced Blair in 1982; Blair returned in 2002, replacing Epstein. Steve Ferrone replaced Lynch in 1995. Scott Thurston (of The Motels) joined as tour guitarist in 1991 and eventually became a permanent member of the band. Petty appeared in the movies FM, Made In Heaven and The Postman and also voiced the character of Elroy “Lucky” Kleinschmidt on the animated TV series King Of The Hill. Member of the Traveling Wilburys. Tom Petty & The Heartbreakers were the subject of the 2007 documentary film Runnin’ Down A Dream, directed by Peter Bogdanovich. One of America’s favorite rock bands of the past five decades; they had wrapped up a 40th Anniversary concert tour shortly before Petty’s sudden death. Also see Mudcrutch. AWARDS: R&R Hall of Fame: 2002 Billboard Century: 2005 9/24/77+ 55 42 G 1 Tom Petty & The Heartbreakers................................................................................................................................. Shelter 52006 6/10/78 23 24 G 2 You’re Gonna Get It! ..................................................................................................................................................