FINAL Rare Epilepsy Landscape Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Common Ribs of Inhibitory Synaptic Dysfunction in the Umbrella of Neurodevelopmental Disorders
Psychology Faculty Publications Psychology 4-28-2018 Common Ribs of Inhibitory Synaptic Dysfunction in the Umbrella of Neurodevelopmental Disorders Rachel Ali Rodriguez University of Nevada, Las Vegas Christina Joya University of Nevada, Las Vegas Rochelle M. Hines University of Nevada, Las Vegas, [email protected] Follow this and additional works at: https://digitalscholarship.unlv.edu/psychology_fac_articles Part of the Neurosciences Commons Repository Citation Rodriguez, R. A., Joya, C., Hines, R. M. (2018). Common Ribs of Inhibitory Synaptic Dysfunction in the Umbrella of Neurodevelopmental Disorders. Frontiers in Molecular Neuroscience, 11 1-23. http://dx.doi.org/10.3389/fnmol.2018.00132 This Article is protected by copyright and/or related rights. It has been brought to you by Digital Scholarship@UNLV with permission from the rights-holder(s). You are free to use this Article in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you need to obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Article has been accepted for inclusion in Psychology Faculty Publications by an authorized administrator of Digital Scholarship@UNLV. For more information, please contact [email protected]. fnmol-11-00132 April 21, 2018 Time: 12:35 # 1 REVIEW published: 24 April 2018 doi: 10.3389/fnmol.2018.00132 Common Ribs of Inhibitory Synaptic Dysfunction in the Umbrella of Neurodevelopmental Disorders Rachel Ali Rodriguez, Christina Joya and Rochelle M. Hines* Neuroscience Emphasis, Department of Psychology, University of Nevada, Las Vegas, Las Vegas, NV, United States The term neurodevelopmental disorder (NDD) is an umbrella term used to group together a heterogeneous class of disorders characterized by disruption in cognition, emotion, and behavior, early in the developmental timescale. -
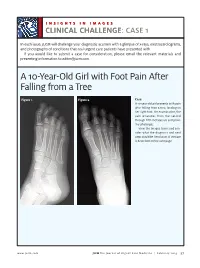
A 10-Year-Old Girl with Foot Pain After Falling from a Tree
INSIGHTS IN IMAGES CLINICAL CHALLENGECHALLENGE: CASE 1 In each issue, JUCM will challenge your diagnostic acumen with a glimpse of x-rays, electrocardiograms, and photographs of conditions that real urgent care patients have presented with. If you would like to submit a case for consideration, please email the relevant materials and presenting information to [email protected]. A 10-Year-Old Girl with Foot Pain After Falling from a Tree Figure 1. Figure 2. Case A 10-year-old girl presents with pain after falling from a tree, landing on her right foot. On examination, the pain emanates from the second through fifth metatarsals and proxi- mal phalanges. View the images taken and con- sider what the diagnosis and next steps would be. Resolution of the case is described on the next page. www.jucm.com JUCM The Journal of Urgent Care Medicine | February 2019 37 INSIGHTS IN IMAGES: CLINICAL CHALLENGE THE RESOLUTION Figure 1. Mediastinal air Figure 1. Differential Diagnosis Pearls for Urgent Care Management and Ⅲ Fracture of the distal fourth metatarsal Considerations for Transfer Ⅲ Plantar plate disruption Ⅲ Emergent transfer should be considered with associated neu- Ⅲ Sesamoiditis rologic deficit, compartment syndrome, open fracture, or vas- Ⅲ Turf toe cular compromise Ⅲ Referral to an orthopedist is warranted in the case of an in- Diagnosis tra-articular fracture, or with Lisfranc ligament injury or ten- Angulation of the distal fourth metatarsal metaphyseal cortex derness over the Lisfranc ligament and hairline lucency consistent with fracture. Acknowledgment: Images courtesy of Teleradiology Associates. Learnings/What to Look for Ⅲ Proximal metatarsal fractures are most often caused by crush- ing or direct blows Ⅲ In athletes, an axial load placed on a plantar-flexed foot should raise suspicion of a Lisfranc injury 38 JUCM The Journal of Urgent Care Medicine | February 2019 www.jucm.com INSIGHTS IN IMAGES CLINICAL CHALLENGE: CASE 2 A 55-Year-Old Man with 3 Hours of Epigastric Pain 55 years PR 249 QRSD 90 QT 471 QTc 425 AXES P 64 QRS -35 T 30 Figure 1. -

Reframing Psychiatry for Precision Medicine
Reframing Psychiatry for Precision Medicine Elizabeth B Torres 1,2,3* 1 Rutgers University Department of Psychology; [email protected] 2 Rutgers University Center for Cognitive Science (RUCCS) 3 Rutgers University Computer Science, Center for Biomedicine Imaging and Modelling (CBIM) * Correspondence: [email protected]; Tel.: (011) +858-445-8909 (E.B.T) Supplementary Material Sample Psychological criteria that sidelines sensory motor issues in autism: The ADOS-2 manual [1, 2], under the “Guidelines for Selecting a Module” section states (emphasis added): “Note that the ADOS-2 was developed for and standardized using populations of children and adults without significant sensory and motor impairments. Standardized use of any ADOS-2 module presumes that the individual can walk independently and is free of visual or hearing impairments that could potentially interfere with use of the materials or participation in specific tasks.” Sample Psychiatric criteria from the DSM-5 [3] that does not include sensory-motor issues: A. Persistent deficits in social communication and social interaction across multiple contexts, as manifested by the following, currently or by history (examples are illustrative, not exhaustive, see text): 1. Deficits in social-emotional reciprocity, ranging, for example, from abnormal social approach and failure of normal back-and-forth conversation; to reduced sharing of interests, emotions, or affect; to failure to initiate or respond to social interactions. 2. Deficits in nonverbal communicative behaviors used for social interaction, ranging, for example, from poorly integrated verbal and nonverbal communication; to abnormalities in eye contact and body language or deficits in understanding and use of gestures; to a total lack of facial expressions and nonverbal communication. -

1 ILAE Classification & Definition of Epilepsy Syndromes in the Neonate
ILAE Classification & Definition of Epilepsy Syndromes in the Neonate and Infant: Position Statement by the ILAE Task Force on Nosology and Definitions Authors: Sameer M Zuberi1, Elaine Wirrell2, Elissa Yozawitz3, Jo M Wilmshurst4, Nicola Specchio5, Kate Riney6, Ronit Pressler7, Stephane Auvin8, Pauline Samia9, Edouard Hirsch10, O Carter Snead11, Samuel Wiebe12, J Helen Cross13, Paolo Tinuper14,15, Ingrid E Scheffer16, Rima Nabbout17 1. Paediatric Neurosciences Research Group, Royal Hospital for Children & Institute of Health & Wellbeing, University of Glasgow, Member of European Reference Network EpiCARE, Glasgow, UK. 2. Divisions of Child and Adolescent Neurology and Epilepsy, Department of Neurology, Mayo Clinic, Rochester MN, USA. 3. Isabelle Rapin Division of Child Neurology of the Saul R Korey Department of Neurology, Montefiore Medical Center, Bronx, NY USA. 4. Department of Paediatric Neurology, Red Cross War Memorial Children’s Hospital, Neuroscience Institute, University of Cape Town, South Africa. 5. Rare and Complex Epilepsy Unit, Department of Neuroscience, Bambino Gesu’ Children’s Hospital, IRCCS, Member of European Reference Network EpiCARE, Rome, Italy 6. Neurosciences Unit, Queensland Children's Hospital, South Brisbane, Queensland, Australia. Faculty of Medicine, University of Queensland, Queensland, Australia. 7. Clinical Neuroscience, UCL- Great Ormond Street Institute of Child Health, London, UK. Department of Clinical Neurophysiology, Great Ormond Street Hospital for Children NHS Foundation Trust, Member of European Reference Network EpiCARE London, UK 8. Université de Paris, AP-HP, Hôpital Robert-Debré, INSERM NeuroDiderot, DMU Innov-RDB, Neurologie Pédiatrique, Member of European Reference Network EpiCARE, Paris, France. 9. Department of Paediatrics and Child Health, Aga Khan University, East Africa. 1 10. Neurology Epilepsy Unit “Francis Rohmer”, INSERM 1258, FMTS, Strasbourg University, France. -

Genetic Mechanisms of Disease in Children: a New Look
Genetic Mechanisms of Disease in Children: A New Look Laurie Demmer, MD Tufts Medical Center and the Floating Hospital for Children Boston, MA Traditionally genetic disorders have been linked to the ‘one gene-one protein-one disease’ hypothesis. However recent advances in the field of molecular biology and biotechnology have afforded us the opportunity to greatly expand our knowledge of genetics, and we now know that the mechanisms of inherited disorders are often significantly more complex, and consequently, much more intriguing, than originally thought. Classical mendelian disorders with relatively simple genetic mechanisms do exist, but turn out to be far more rare than originally thought. All patients with sickle cell disease for example, carry the same A-to-T point mutation in the sixth codon of the beta globin gene. This results in a glutamate to valine substitution which changes the shape and the function of the globin molecule in a predictable way. Similarly, all patients with achondroplasia have a single base pair substitution at nucleotide #1138 of the FGFR3 gene. On the other hand, another common inherited disorder, cystic fibrosis, is known to result from changes in a specific transmembrane receptor (CFTR), but over 1000 different disease-causing mutations have been reported in this single gene. Since most commercial labs only test for between 23-100 different mutations, interpreting CFTR mutation testing is significantly complicated by the known risk of false negative results. Many examples of complex, or non-Mendelian, inheritance are now known to exist and include disorders of trinucleotide repeats, errors in imprinting, and gene dosage effects. -

1 Long-Read Genome Sequencing for the Diagnosis Of
bioRxiv preprint doi: https://doi.org/10.1101/2020.07.02.185447; this version posted September 14, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Long-read genome sequencing for the diagnosis of neurodevelopmental disorders Susan M. Hiatt1, James M.J. Lawlor1, Lori H. Handley1, Ryne C. Ramaker1, Brianne B. Rogers1,2, E. Christopher Partridge1, Lori Beth Boston1, Melissa Williams1, Christopher B. Plott1, Jerry Jenkins1, David E. Gray1, James M. Holt1, Kevin M. Bowling1, E. Martina Bebin3, Jane Grimwood1, Jeremy Schmutz1, Gregory M. Cooper1* 1HudsonAlpha Institute for Biotechnology, Huntsville, AL, USA, 35806 2Department of Genetics, University of Alabama at Birmingham, Birmingham, AL, USA, 35924 3Department of Neurology, University of Alabama at Birmingham, Birmingham, AL, USA, 35924 *[email protected], 256-327-9490 Conflicts of Interest The authors all declare no conflicts of interest. 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.07.02.185447; this version posted September 14, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Abstract Purpose Exome and genome sequencing have proven to be effective tools for the diagnosis of neurodevelopmental disorders (NDDs), but large fractions of NDDs cannot be attributed to currently detectable genetic variation. This is likely, at least in part, a result of the fact that many genetic variants are difficult or impossible to detect through typical short-read sequencing approaches. -

Research Quarterly
Research Quarterly A dream you dream alone is only a dream. A dream you dream together is reality. – Yoko Ono In this issue of the Quarterly, we highlight some of the partnerships our research team has developed to make a bigger impact for people with epilepsy. Our research covers the entire spectrum of discovery – from idea to market. We cannot do it alone. On page 2, we provide updates on the Rare Epilepsy Network (REN), a coalition of nearly 30 different organizations working together to facilitate research that improves the outcomes of those living with rare epilepsies. On page 3, clinicians from Thomas Jefferson University Hospital in Philadelphia, PA reflect on how the Epilepsy Foundation’s support early on in their career encouraged them to pursue research within the field. Supporting the development of early career investigators is done in proud partnership with the American Epilepsy Society (AES). This collaborative effort ensures that we can pool our resources, reduce administrative cost and maximize impact. Supporting our professional workforce is one of the ways in which we can ensure that the best and the brightest are tackling the challenges that our community face. We could not do this without our partners at AES. On page 5, we provide staff reports on the conferences attended this past quarter from the Nonprofits Forum at the National Institutes of Health, to meeting new companies at Bio Conference, to facilitating discussions on how medical devices can impact SUDEP and epilepsy care with the Food and Drug Administration. We are creating a research environment in which partnerships spur innovation and exciting discoveries to end epilepsy. -

Segmental Overvekst Og Vaskulærmalformasjoner V02
2/1/2021 Segmental overvekst og vaskulærmalformasjoner v02 Avdeling for medisinsk genetikk Segmental overvekst og vaskulærmalformasjoner Genpanel, versjon v02 * Enkelte genomiske regioner har lav eller ingen sekvensdekning ved eksomsekvensering. Dette skyldes at de har stor likhet med andre områder i genomet, slik at spesifikk gjenkjennelse av disse områdene og påvisning av varianter i disse områdene, blir vanskelig og upålitelig. Disse genetiske regionene har vi identifisert ved å benytte USCS segmental duplication hvor områder større enn 1 kb og ≥90% likhet med andre regioner i genomet, gjenkjennes (https://genome.ucsc.edu). Vi gjør oppmerksom på at ved identifiseringav ekson oppstrøms for startkodon kan eksonnummereringen endres uten at transkript ID endres. Avdelingens websider har en full oversikt over områder som er affisert av segmentale duplikasjoner. ** Transkriptets kodende ekson. Ekson Gen Gen affisert (HGNC (HGNC Transkript Ekson** Fenotype av symbol) ID) segdup* ACVRL1 175 NM_000020.3 2-10 Telangiectasia, hereditary hemorrhagic, type 2 OMIM ADAMTS3 219 NM_014243.3 1-22 Hennekam lymphangiectasia- lymphedema syndrome 3 OMIM AKT1 391 NM_005163.2 2-14 Cowden syndrome 6 OMIM Proteus syndrome, somatic OMIM AKT2 392 NM_001626.6 2-14 Diabetes mellitus, type II OMIM Hypoinsulinemic hypoglycemia with hemihypertrophy OMIM AKT3 393 NM_005465.7 2-14 Megalencephaly-polymicrogyria- polydactyly-hydrocephalus syndrome 2 OMIM file:///data/SegOv_v02-web.html 1/7 2/1/2021 Segmental overvekst og vaskulærmalformasjoner v02 Ekson Gen Gen affisert (HGNC (HGNC -

Complete Loss of CASK Causes Severe Ataxia Through Cerebellar Degeneration
Complete loss of CASK causes severe ataxia through cerebellar degeneration Paras Patel Fralin Biomedical Research Institute at VTC Julia Hegert Orlando Health Corp Ingrid Cristian Orlando Health Corp Alicia Kerr National Eye Institute Leslie LaConte Fralin Biomedical Research Institute at VTC Michael Fox Fralin Biomedical Research Institute at VTC Sarika Srivastava Fralin Biomedical Research Institute at VTC Konark Mukherjee ( [email protected] ) Fralin Biomedical Research Institute at VTC https://orcid.org/0000-0002-6922-9554 Research article Keywords: CASK, MICPCH, neurodegeneration, X-linked, X-inactivation, cerebellum, ataxia Posted Date: May 4th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-456061/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/34 Abstract Background: Heterozygous loss of X-linked genes like CASK and MeCP2 (Rett syndrome) causes neurodevelopmental disorders (NDD) in girls, while in boys loss of the only allele of these genes leads to profound encephalopathy. The cellular basis for these disorders remains unknown. CASK is presumed to work through the Tbr1-reelin pathway in neuronal migration. Methods: Here we report clinical and histopathological analysis of a deceased 2-month-old boy with a CASK-null mutation. We rst analyze in vivo data from the subject including genetic characterization, magnetic resonance imaging (MRI) ndings, and spectral characteristics of the electroencephalogram (EEG). We next compare features of the cerebellum to an-age matched control. Based on this, we generate a murine model where CASK is completely deleted from post-migratory neurons in the cerebellum. Results: Although smaller, the CASK-null human brain exhibits normal lamination without defective neuronal differentiation, migration, or axonal guidance, excluding the role of reelin. -

Sensory Deficits in a Mouse Model of Christianson Syndrome Tarheen
Sensory Deficits in a Mouse Model of Christianson Syndrome Tarheen Fatima Department of Physiology McGill University, Montreal April 2019 A thesis submitted to McGill University in partial fulfillment of the requirements of the degree of Master of Science. © Tarheen Fatima 2019 Abstract Christianson Syndrome (CS) is a recently characterized X-linked neurodevelopmental disorder caused by loss-of-function mutations in the gene slc9a6, encoding the endosomal Na+/H+ Exchanger 6 (NHE6). The disorder is associated with developmental delay, intellectual disability, loss of motor coordination, mutism, ataxia, epilepsy, as well as autistic features. In addition to these symptoms, CS patients exhibit elevated pain thresholds to noxious stimuli as well as discomfort at normally innocuous stimuli. The underlying causes of these sensory deficits are yet to be determined. Hence, this study aims at understanding how loss-of-function of NHE6 affects transmission and processing of pain. To this end, we examined the expression of NHE6 in peripheral and central neurons implicated in sensation and interpretation of pain. Additionally, we characterized the nociceptive behaviour of NHE6 knockout (KO) mice using a battery of behaviour tests. Our immunohistochemical experiments demonstrate that NHE6 is highly expressed in nociceptive, small-diameter dorsal root ganglia (DRG) neurons. Moreover, mice lacking NHE6 display decreased responses to noxious mechanical, thermal and chemical stimuli but are more responsive to noxious cold than wild-type littermates. Interestingly, immunohistochemical characterization of DRG tissue from aged NHE6 null mice indicates a decrease in some neuronal subsets suggesting cell death. Finally, using light brush-induced Fos activation in the dorsal horn, we found that the spinal processing of innocuous stimuli is not different between wildtype and NHE6 knockout mice. -

What Is Christianson Syndrome? by Dr
What is Christianson Syndrome? By Dr. Eric Morrow Christianson syndrome is a genetic disorder that affects brain development. It is an “X-linked” disorder (see “Why is CS found only in boys?” section for a description of what X-linked means). Symptoms of CS usually appear in infancy and include epilepsy and intellectual disability. Although the exact prevalence of Christianson syndrome is unknown, a genetic study by Tarpey and colleagues identified Christianson Syndrome in 2 of about 200 families (about 1% of families with apparent X-linked developmental disabilities). This was among the most common genetic change in X-linked forms of intellectual disability. What are the causes of Christianson syndrome? Christianson syndrome is caused by changes in the gene, SLC9A6. Genes are important because they code for proteins, which carry out essential processes in the cells of our bodies. The SLC9A6 gene codes for the NHE6 protein. NHE6 is part of a family of proteins that are sodium (Na+)/hydrogen (H+) exchangers. The NHE6 protein is found in endosomes, which take up molecules to be destroyed or recycled for other processes in our body. NHE6 makes sure that these endosomes have the correct intra-endosomal acid level by letting in Na into the endosome and getting rid of hydrogen, which is the acid molecule. The acid level regulates protein turnover. My analogy our stomach acid, the acidity in endosomes helps degrade cellular proteins. These genetic changes in SLC9A6 cause the NHE6 protein to not work properly. You may have heard words such as “loss-of-function”, “truncation” or “frame- shift”, which describe how the NHE6 protein cannot be made in the cells to carry out these processes in the endosome. -

Towards Therapy for Batten Disease
Towards therapy for Batten disease Mariana Catanho da Silva Vieira MRC Laboratory for Molecular Cell Biology University College London PhD Supervisor: Dr Sara E Mole A thesis submitted for the degree of Doctor of Philosophy University College London September 2014 Declaration I, Mariana Catanho da Silva Vieira, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. 2 Abstract The gene underlying the classic neurodegenerative lysosomal storage disorder (LSD) juvenile neuronal ceroid lipofuscinosis (JNCL) in humans, CLN3, encodes a polytopic membrane spanning protein of unknown function. Several studies using simpler models have been performed in order to further understand this protein and its pathological mechanism. Schizosaccharomyces pombe provides an ideal model organism for the study of CLN3 function, due to its simplicity, genetic tractability and the presence of a single orthologue of CLN3 (Btn1p), which exhibits a functional profile comparable to its human counterpart. In this study, this model was used to explore the effect of different mutations in btn1 as well as phenotypes arising from complete deletion of the gene. Different btn1 mutations have different effects on the protein function, underlining different phenotypes and affecting the levels of expression of Btn1p. So far, there is no cure for JNCL and therefore it is of great importance to identify novel lead compounds that can be developed for disease therapy. To identify these compounds, a drug screen with btn1Δ cells based on their sensitivity to cyclosporine A, was developed. Positive hits from the screen were validated and tested for their ability to rescue other specific phenotypes also associated with the loss of btn1.