Guidance Notes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bakery Price List
BAKERY GOODS ROLLS PRICE SLICED BREAD PRICE MORNING ROLL £0.31 HOMESTYLE WHITE 800G £2.00 WHEATEN ROLL £0.36 RICH FRUIT LOAF £2.45 BARM £0.35 MULTISEED 800G £2.38 BURGER ROLL £0.35 COUNTRY COARSE 400G £1.90 GRANARY ROLL £0.36 CRUSTY 400G £1.90 ROUGH W/MEAL ROLL £0.35 GLENCRUST 400G £2.00 CRACKED WHEAT ROLL £0.35 WHEATMEAL 400G £1.76 TRADITIONAL ROLL £0.32 BERRYMEAL 400G £2.00 CHEESY BAP £0.42 CRUSTY 800G £2.80 POPPYSEED FINGER £0.35 RUSTIC 800G £2.95 WHITE DINNER ROLL £0.26 RUSTIC 400G £2.00 BROWN DINNER ROLL £0.26 HERB & ONION 400G £2.05 FYNE HERB & ONION DINNER ROLL £0.28 BROWN SANDWICH LOAF 800G £2.00 WHITE SUB £0.45 SOFTIE £0.35 NEW YORK HOT DOG ROLL £0.40 TEABREAD BRIOCHE ROLL £0.62 OATIE SUB £0.45 PLAIN COOKIE £0.40 HERB & ONION SUB £0.45 FARM SCONE £0.78 LARGE POPPYSEED SUB £0.45 COTTAGE SCONE £0.50 SULTANA SCONE £0.50 BREAD POTATO SCONE £0.33 CRUMPET £0.50 CRUSTY 400G £1.90 HOT X BUN £0.42 GLENCRUST 400G £2.00 PANCAKE £0.48 BERRYMEAL 400G £2.00 CROISSANT £0.56 BAGUETTE £2.00 WHEATMEAL 400G £1.76 COUNTRY COARSE 400G £1.90 HERB & ONION 400G £2.05 RICH FRUIT LOAF £2.45 DO-NUTS RUSTIC 800G £2.95 CRUSTY 800G £2.80 SUGAR RING DO-NUT £0.54 BROICHE LOAF 400G £1.90 ICED DO-NUT £0.70 RUSTIC 400G £2.00 JAM BALL DO-NUT £0.70 PASTRIES PRICE CONFECTIONERY SMALL PRICE CAKES APPLE TART S/CRUST £2.65 CHOCOLATE & MARZIPAN £0.86 LOG RHUBARB TART S/CRUST £2.65 FRENCH CAKE £0.80 DANISH PASTRY £0.94 TOFFEE SLICE £0.82 APPLE TURNOVER £1.06 ICED GINGER SLICE £0.80 CHELSEA BUN £0.94 FERN CAKE £0.72 APPLE & CUSTARD DANISH £1.06 PINEAPPLE PEAK £0.78 RASPBERRY -

The Chemistry of Beef Flavor - Executive Summary Prepared for the National Cattlemen’S Beef Association M
® Funded by the Beef Checkoff The Chemistry of Beef Flavor - Executive Summary Prepared for the National Cattlemen’s Beef Association M. Susan Brewer, Ph.D., Department of Food Science and Human Nutrition, University of Illinois, December, 2006 The beef industry is continually working to satisfy consumer expectations for dependable, high quality beef products at a reasonable cost to producer, packer, processor and retailer. Flavor and tenderness are the sensory traits that affect consumer acceptance of beef the most; therefore, it is vital that both traditional and new beef systems assure consistently tender products with acceptable flavor. — Kerth et al., 1995 between some of the more common volatiles in beef and “Flavor” results from the combination their respective flavors is shown in Table 1. Beef flavor, which of basic tastes (sweet, sour, bitter, salt and umami) derived develops when heat is applied, depends on the amounts from water-soluble compounds and odor derived from a and proportions of precursor compounds present. Meat is myriad of substances present in the food product from the composed of water, proteins, lipids, carbohydrates, minerals onset or derived via various reactions. The flavors and aromas and vitamins. Of these, proteins, lipids and carbohydrates associated with beef are generally those that develop during play primary roles in flavor development because they include heating. When water-soluble substances derived from precursor numerous compounds which are capable of developing into compounds dissolve in the saliva, they bind to the taste buds important flavor precursors when heated (Spanier and Miller, and stimulate a response that is perceived in the brain. -

Counter Price List 2021
MACDONALD BROS. LTD 6/8 Bonnethill Road Pitlochry PH16 5BS Tel 01796 472047 12 Bank Street Aberfeldy PH15 2BB Tel 01887 820310 Email [email protected] www.macdonald-bros.co.uk. Counter Price List 2021 1 ABERDEEN ANGUS BEEF per Kilo FRESH SCOTTISH LAMB per kilo FILLET STEAK £42.98 LAMB FILLET £33.04 WHOLE BEEF FILLET £42.98 DICED LEG OF LAMB £18.25 M/C BEEF FILLET £49.50 LEG OF LAMB ROLLED £18.28 SIRLOIN STEAK £31.00 BONELESS LAMB STEAKS £18.34 ROLLED SIRLOIN £28.00 GIGOT CHOPS £14.48 RIBEYE STEAKS £32.00 LAMB RUMP £18.34 VEAL ESCALLOPE £27.22 CENTRE-CUT LEG OF LAMB £16.20 ROLLED RIBROAST £21.10 LOIN OF LAMB £15.50 FRY STEAK £22.34 SADDLE OF LAMB BONE-IN £15.50 SIRLOIN ON-BONE £22.42 LEG OF LAMB £15.90 T-BONE STEAK £24.46 DOUBLE-LOIN CHOPS £16.28 RIBROAST ON-BONE £19.48 LAMB CUTLETS / RACK OF LAMB £16.28 SILVERSIDE £13.80 DICED SHOULDER LAMB £14.26 TOPSIDE £13.98 STEWING LAMB £14.26 ROUND STEAK £14.25 SHOULDER LAMB CHOPS £10.18 MINUTE STEAK £12.22 LAMB KEBABS each £3.00 BEEF OLIVES £10.45 SHOULDER LAMB ROLLED £12.22 SPALEBONE £10.85 MINCED SHOULDER LAMB £14.08 SALMON-CUT STEAK £10.85 NECK OF LAMB £7.65 BRISKET £11.20 LAMB KIDNEY £6.73 SHOULDER STEAK £12.80 LAMB LIVER £6.78 STEAK MINCE £11.20 LAMB SHANKS £6.81 SHIN OF BEEF/HOUGH £9.32 FLANK OF LAMB £6.73 SHORT RIB £8.52 LAMB HEART £5.68 OX TONGUE £6.20 LAMB BONES £2.20 OX KIDNEY £5.98 OX TAIL £7.20 VENISON & GAME per kilo OX LIVER £5.98 VENISON FILLET £29.40 DOG MINCE £3.92 VENISON STEAK £19.86 BEEF BONES £2.20 VENISON SADDLE £17.30 RACK OF VENISON £18.76 FRESH SCOTTISH PORK per kilo -
Boneless Beef Clod Ferraro's Boneless $1099
AT BOTH OF OUR LOCATIONS!!! 664 Grand Avenue, New Haven Mon. - Fri. 8:30-6:00 | Sat. 8:00-6:00 Closed Sunday Family Owned & 181 Boston Post Road, Madison Operated for over 60 years Tues - Fri. 10:00-6:00 | Sat. 9:00-5:00 | Closed Sunday & Monday SALE IN EFFECT: We Accept Visa, THURSDAY,AUG 15TH Mastercard,AMEX, WE RESERVE THE RIGHT www.FerraroMarket.com WIC & EBT THROUGH Find us on Facebook WEDNESDAY,AUG 21ST, 2019 TO LIMIT QUANTITIES! CUT TO BONELESS BEEF FERRARO’S FAMOUS BONELESS BEEF ORDER BEEF LONDON ITALIAN STYLE RIB EYE TENDERLOIN BROIL PORKETTA (FILET MIGNON) STEAKS ROAST BUTT $ 99 WHOLE PORTION $ 99 $ 99 $ 99 $ 99 WHOLE 6 LB OR WOW! 2 LB 10 LB 8 LB 3 1/2 PORTION LB READY TO COOK BONELESS BEEF CLOD FERRARO’S W/ FRESH FRESH CHICKEN CHICKEN BEEF VEGGIES MEATLOAF MIX BONE-IN PEPPERS, ONIONS & SPICES STEAKS KABOBS ¢ $ 99 $ 99 $ 99 CHICKEN 89 LB FAMILY PACK 1 3 LB THIGHS 5 LB LB THIN SLICED DOLLAR STRETCHER! BONELESS FRESH PORK PORK WHOLE PORK BABY BACK CUTLETS BONELESS SIRLOIN HAND SLICED ON PREMISES PORK LOIN CHOP RIBS $ 99 $ 79 $ 99 $ 99 GREAT STEAL! PRICE! 3 1 LB LB 3 LB 1 LB FERRARO’S FERRARO’S BEEF PEPPER STUFFED FRESH BONELESS ITALIAN STEAK BURGERS PORK CHOPS SAUSAGE CHICKEN ¢ *ITALIAN, FLORENTINE, CORNBREAD* HOT 59 OR LB $ 99 99 SWEET $ 99 MEATY $ FAMILY 3 LB PACK 2 LB DRUMSTICKS 3 LB FERRARO’S FRANKS FERRARO’S KITCHEN TENDER SWEET FRESH, MEATY 3 POUND PKGS SCROD • BEEF SKINLESS PASTA WITH SWEET FRANKS BROCCOLI YOUR CHOICE FILLET • BEEF GEORGIA RABE $ 49 HOTS YOUR CHOICE 3 MUSSELS ITALIAN LB $ 99 $ 99 SAUSAGE 5 2 LBS /$ 00 LB 3 11 EA & PEPPERS FRESH LIVE FERRARO’S BAR S FERRARO’S FRESH PASTA MAINE SEAFOOD JUMBO RICOTTA LOBSTER STUFFED CLAMS FRANKS CAVATELLI EVERDAY $ 99 $ 99 LOW $ 00 $ 99 3 5 PRICE! POUNDS! 12OZ LB 2 FOR 3 4 1 EA EA TASTE LIKE LOBSTER GREAT DEAL! FERRARO’S KITCHEN FRESH, TENDER OLD SCHOOL DISHES SLICED TASTE MONK LIKE HOT DOGS TOP NECK PORK BACON! AND YOUR FILLET SHOULDER CHOICE CLAMS POTATOES $ 99 2 LB 10 $ 99 $ 99 ¢ CHICKEN 5 LB SMOKED 99 FOR 3 LB CACCIATORE AT BOTH OF OUR LOCATIONS!!! 664 Grand Avenue, New Haven Mon.-Fri. -

Meat and Muscle Biology™ Introduction
Published September 7, 2017 Meat and Muscle Biology™ Light Source Influences Color Stability and Lipid Oxidation in Steaks from Low Color Stability Beef Triceps brachii Muscle Jade V. Cooper1, Surendranath P. Suman2, Bryon R. Wiegand1, Leon Schumacher3, and Carol L. Lorenzen1* 1Division of Animal Sciences, University of Missouri, Columbia, MO 65211, USA 2Department of Animal and Food Sciences, University of Kentucky, Lexington, KY 40546, USA 3Department of Agricultural Systems Management, University of Missouri, Columbia, MO 65211, USA *Corresponding author. Email: [email protected] (C. L. Lorenzen) Abstract: Color of retail fresh meat is one of the most important quality attributes affecting purchasing decisions for con- sumers. The objective of this study was to evaluate the impact of different light sources on surface color and lipid oxidation during retail display of fresh steaks from beef Triceps brachii (TB), a muscle with low color and lipid oxidative stabilities. Steaks (n = 12) from 20 TB muscles were overwrapped with oxygen-permeable polyvinyl chloride, and assigned to one of three lighting treatments, i.e., high UV fluorescent (HFLO), low UV fluorescent (FLO), and light emitting diode (LED), in temperature-controlled deli cases. Steaks were removed on retail display d 1, 3, 5, and 7 for evaluating instrumental color (L*, a*, and b* values), surface myoglobin redox forms, metmyoglobin reducing ability, and lipid oxidation. Surface redness (a* values) of TB steaks decreased (P < 0.05) during retail display. Light source influenceda * values, with HFLO-displayed steaks having higher (P < 0.05) a* values than steaks exposed to both FLO and LED light sources. Oxymyoglobin levels were higher (P < 0.05) for TB steaks displayed under HFLO lights than those displayed under FLO (on d 3 and 7) or LED (on d 5 and 7) lights. -

Effect of Packaging Type and Storage Temperature on the Quality
─── Food Technology ─── Effect of packaging type and storage temperature on the quality characteristics of beef longissimus lumborum and triceps brachii muscles aged for extended storage postmortem Amie G. Osterhout, C. Chad Carr, D. Dwain Johnson Department of Animal Sciences, University of Florida, Gainesville, USA Abstract Keywords: Introduction. The purpose of the study was to Triceps brachii quantify the impact of packaging type, storage temperature, Beef and length of postmortem aging to consistently increase Drybag palatability and color stability of Longissimus lumborum Dry aging (LL) and TBmuscles that represent the bulk of today’s Extended aging supply. Materials and methods. Paired longissimus lumborum (LL) and triceps brachii (TB) muscles were selected from 27 and 54 A-maturity beef carcasses with marbling scores of Slight 50 to Small 50 at grading. One Article history: muscle from each pair was stored at 0 and 4 °C, Received 22.03.2019 respectively, for 21, 32, or 42 days for LL, and 21, 28, or Received in revised form 35 days for TB, in one of three packaging options; 16.07.2019 DryBag®, traditional vacuum-bag, or no bag. Accepted 30.09.2019 Result and discussion. Saleable yields were similar forDryBag®and traditional dry agedsubprimals. Aging temperature and packaging type had little impact on any quality parameters evaluated. Steaks from LL muscles aged Corresponding author: for 42 days had lower shear force values and more tender trained sensory panel values than steaks from LL aged for C. Chad Carr 21 days, although the actual difference was marginal. E-mail: [email protected] Tenderness of steaks from TB muscles was similar regardless of aging. -
Lunch Menu January 2015
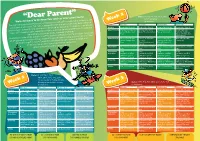
“Dear Parent” Dates: 2-12/1, 2/2, 2/3, 23/3, 27/4, 18/5*, 8/6,29/6 Welcome back to the New Year and our new school menu. Week 2 (v) Vegetarian This year sees a big change in our school meal provision with all P1-3 pupils being entitled to a free Monday Tuesday Wednesday Thursday school lunch in all schools. The menu is designed to offer all pupils a nutritionally balanced lunch Starter Soup of the Day (v) Soup of the Day (v) Soup of the Day (v) Soup of the Day (v) which includes some of their favourite dishes. We would encourage all P1-3 pupils to come and try! Main Dish (1) Macaroni Cheese (v), Stovies with Home Chicken Curry with Roast Beef and Jacket Wedges and Made Bread & Carrots Rice & Sweetcorn Yorkshire Pudding, To give all P1-3 pupils a wider experience of tastes and foods we will put all items on the plate and this might encourage new tastes, especially vegetables! Peas Mashed Potato and Green Beans Packed lunch option will be available for all P1-3 pupils on a Friday but to help us reduce food waste Main Dish (2) Fillet of Fish Fingers, Cheese and Tomato Cheese and Onion Baked Potato with we are gathering information via the schools of those interested in taking up this option on a Friday. Jacket Wedges and Pizza (v), Bridie (v) with Baby Cheese (v) Peas Roast Potatoes and Boiled Potatoes & We haven’t forgotten our P4-7 pupils they will still get the same options at the price of £1.80, less than a cost of some supermarket sandwiches! Carrots Sweetcorn All our staff look forward to welcoming you! Vegetables Mixed Salad available -

Name, a Novel
NAME, A NOVEL toadex hobogrammathon /ubu editions 2004 Name, A Novel Toadex Hobogrammathon Cover Ilustration: “Psycles”, Excerpts from The Bikeriders, Danny Lyon' book about the Chicago Outlaws motorcycle club. Printed in Aspen 4: The McLuhan Issue. Thefull text can be accessed in UbuWeb’s Aspen archive: ubu.com/aspen. /ubueditions ubu.com Series Editor: Brian Kim Stefans ©2004 /ubueditions NAME, A NOVEL toadex hobogrammathon /ubueditions 2004 name, a novel toadex hobogrammathon ade Foreskin stepped off the plank. The smell of turbid waters struck him, as though fro afar, and he thought of Spain, medallions, and cork. How long had it been, sussing reader, since J he had been in Spain with all those corkoid Spanish medallions, granted him by Generalissimo Hieronimo Susstro? Thirty, thirty-three years? Or maybe eighty-seven? Anyhow, as he slipped a whip clap down, he thought he might greet REVERSE BLOOD NUT 1, if only he could clear a wasp. And the plank was homely. After greeting a flock of fried antlers at the shevroad tuesday plied canticle massacre with a flash of blessed venom, he had been inter- viewed, but briefly, by the skinny wench of a woman. But now he was in Rio, fresh of a plank and trying to catch some asscheeks before heading on to Remorse. I first came in the twilight of the Soviet. Swigging some muck, and lampreys, like a bad dram in a Soviet plezhvadya dish, licking an anagram off my hands so the ——— woundn’t foust a stiff trinket up me. So that the Soviets would find out. -

FSIS Directive 10010.1 Rev. 3
FSIS DIRECTIVE 10,010.1 Rev. 3 Verification Activities for Escherichia coli O157:H7 in Raw Beef Products TABLE OF CONTENTS Chapter I – General What Raw Ground Beef Products does FSIS sample? What Raw Ground Beef and Patty Components does FSIS sample? What Products are not subject to FSIS sampling? How is a “lot” defined? What is a “positive” result? What is a “presumptive positive” result? What are the Sampling Project Numbers for E. coli O157:H7 testing? Chapter II – IPP Responsibilities for Collecting and Submitting Samples How do I put on my gloves? How do I request sampling supplies? What do I need to know about notifying establishment management about collecting a sample? When do I collect a sample? Do I collect a fresh or frozen sample? What do I do if I receive FSIS Form 10,210-3 requesting samples of ground beef (MT43), trim (MT50), and components other than trim (MT54)? How do I determine the intended use of the product? How do I collect a ground beef sample and how much product do I need to collect for a ground beef sample? Do I collect a ground beef sample that contains poultry or other meat ingredients? What do I need to do if the establishment requests the use of alternative procedures for sampling a lot of ground beef? How do I collect beef manufacturing trimmings samples using the N60 method? How do I select ground beef components other than beef manufacturing trimmings for sampling? FSIS Directive 10,010.1 How do I collect samples of components other than trimmings? How do I collect samples of ammoniated beef products? How do I collect samples of bench trim? Chapter III – FSIS Actions After a Positive FSIS or Another Federal or State Entity Sample Result What do I do when I am notified of an FSIS presumptive E. -

Meat Seafood
MEAT SEAFOOD Category Item Description Pack Size BACON 38064 FAR BACON SINGLE SLI 18/22 NORTHEAST 1 15 LB BACON 38123 TYS BACON SINGLE SLICE CC 18/22 1 15 LB BACON 38458 HAT BACON SLI PREMIUM 24 1 LB BACON 38820 HAT BACON SLI LAYOUT 18/22 CHEF PLEASER 1 15 LB BACON 38823 HAT BACON SLI LAYOUT 14/18 CHEF PLEASER 1 15 LB BACON 38831 HAT BACON SLAB SLI THICK 1 15 LB BACON 38940 TYS BACON SLI LAYOUT FLAT PACK 1 15 LB BACON PRECOOKED 38001 FAR BACON SLI CKD ROUND 1 192 CT BACON PRECOOKED 38006 FAR BACON SLI CKD THICK 300 CT "DELUXE" 1 5.4 LB BACON PRECOOKED 38008 HOR BACON SLI CKD ROUND 1 192 CT BACON PRECOOKED 38020 FAR BACON SLI CKD 300 CT 1 300 CT BACON PRECOOKED 38023 FAR BACON SLI CKD 300 CT 1 300 CT BACON PRECOOKED 38024 FAR BACON CKD (CHIPS) 2 5 LB BACON PRECOOKED 38127 TYS BACON SLI CKD THIN HICKORY SMK 1 3.75 BACON PRECOOKED 38131 TYS BACON SLI CKD ROUND 192 CT 1 192 CT BACON PRECOOKED 38148 TYS BACON SLI CKD THICK 300 CT 1 5.4 LB BACON PRECOOKED 38837 FAR BACON CANADIAN 4 3.2LB A BACON PRECOOKED 38880 FAR BACON CANADIAN 4 4 LB AV BEEF ANGUS - FRESH 38613 PAC BEEF RIB EYE CH -ANGUS BLACK CAN FRZ 5 PC BEEF BOXED FRESH 28500 IBP BEEF TOP ROUND CH 1 PC BEEF BOXED FRESH 28502 IBP BEEF TOP ROUND INSIDE 1 PC BEEF BOXED FRESH 28520 PAC BEEF RIB EYE CH 12/14 1 PC BEEF BOXED FRESH 28522 IBP BEEF RIB EYE NR 12/14 1 PC BEEF BOXED FRESH 28540 PAC BEEF FLANK STEAK PEELED 1 2 PC BEEF BOXED FRESH 28547 IBP BEEF TENDERLOIN PEELED 5/UP 2 PC BEEF BOXED FRESH 28551 IBP BEEF STRIP LOIN NR 1X1 1 PC BEEF BOXED FRESH 28554 IBP BEEF STRIP LOIN NR 0X1 BNLS -

The HOTEL BUTCHER, GARDE MANGER and CARVER
TH EL BUTCHER, GARDE MANAGER AND CARVER: SUGGESTIONS... Frank Rivers m ed by Google The HOTEL BUTCHER, GARDE MANGER and CARVER By FRANK RIVERS Suggestions for the Buying, Handling, Sale, and Service of Meats, Poultry and Fish for Hotels, Restaurants, Clubs, and Institutions An Expression of the Practical Experience of One Who has Spent Thirty Years in All Branches of Kitchen, Pantry and Store- room Work; Also as Steward and Buyer. The Book Supplemented with Gleanings from the pages of THE HOTEL MONTHLY. Copyright 1916, by Frank Rivers PUBLISHED BY THE HOTEL MONTHLY PRESS 443 SOUTH DEARBORN STREET, CHICAGO, ILL. : ' • - ; : i To the Header If we are to work well, we must think well. This book may not accomplish all the prac- tical ends possible, but it is hoped it will help the reader to think and work along right lines. Those who think right may be best trusted to do right. I wi>h to thank and acknowledge my indebt- edness for information and courtesies received by me in compiling this book from the following firms and persons: The Morris Packing Co., Chicago. The Sherman Hotel Co., Chicago. The Hotel Monthly, Chicago. Mr. John Tellman, St. Louis. Albert Pick & Co., Chicago. W. M. Walker & Co.. fish dealers. Chicago. Armour Packing Co., Chicago. Magner-Winslow Provision Co., Chicago. Irwin Bros., meat dealers, Chicago. Mr. L. Wilson, Harvey system, Chicago. J. L. Oxley & Co., veal and poultry, Chicago. Cohen & Co., Chicago. John G. Neumeister Co., Chicago. Fuaxk Rivkks. J L 6 -I 7. 7 THE HOTEL BUTCHER, GARDE MANGER £ CARVER SUGGESTIONS FOR THE BUYING, HANDLING, SALE, AND SERVICE OF MEATS FOR HOTELS, RESTAURANTS, CLUBS AND INSTITUTIONS-^ Frank Rivers INTRODUCTORY REMARKS Generally the chef does the butchering in hotels I have made a life-time study of meats and that do not employ a professional butcher; or the service of meat foods in lintels, restaurants he will have it done by one of his cooks that and clubs, and have attained a degree of profi- has a special fitness or knowledge of the work. -

Meat and Muscle Biology™
Meat and Muscle Biology™ Processing Characteristics, Composition, Shelf-life, and Sensory Attributes of Beef Bacon Manufactured From Seven Value-Added Cuts of Beef Sebastian Chalupa-Krebzdak and Benjamin M. Bohrer* Department of Food Science, University of Guelph, Guelph, Ontario, Canada, N1G 2W1 *Corresponding author. Email: [email protected] (Benjamin M. Bohrer) Abstract: The purpose of this study was to evaluate the influence of different beef cuts on their potential for adding value by assessing processing characteristics, composition, shelf-life, and sensory attributes of these cuts as beef bacon. Six briskets (Institutional Meat Purchase Specification [IMPS]#120), 6 clod hearts (IMPS#114E; divided horizontally into 2 halves: silver-skin side and non–silver-skin side), 6 flanks (IMPS#193), 6 outside flats (IMPS#171B), and 7 short plates (IMPS#121A; cut into a deboned short-rib half and navel half) were sourced commercially from separate Canadian qual- ity grade AA beef carcasses. Data for processing yields, composition, and image analysis were analyzed as a generalized linear mixed model with fixed effect of cut and random effect of replication nested within block (processing group). Sensory data collected using a trained sensory panel were analyzed in the same manner, with an additional fixed effect of storage day and additional random effects of session and panelist. Rested pump uptake, which was targeted at 20%, was not different (P = 0.21) among cuts; however, smokehouse cook yield differed (P < 0.01) among cuts, with heavier cuts (brisket, plate cuts, and outside flat) generally having greater yields compared with lighter cuts (clod cuts and flank). As expected, composition of bacon slices was affected (P < 0.01) by cut, with leaner cuts (clod cuts, flank, and outside flat) having greater moisture, lower lipid levels, and greater protein compared with fatter cuts (brisket and plate cuts).