Earliest Complete Hominin Fifth Metatarsal-Implications for the Evolution of the Lateral Column of the Foot
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
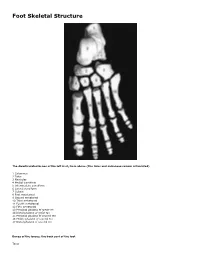
Skeletal Foot Structure
Foot Skeletal Structure The disarticulated bones of the left foot, from above (The talus and calcaneus remain articulated) 1 Calcaneus 2 Talus 3 Navicular 4 Medial cuneiform 5 Intermediate cuneiform 6 Lateral cuneiform 7 Cuboid 8 First metatarsal 9 Second metatarsal 10 Third metatarsal 11 Fourth metatarsal 12 Fifth metatarsal 13 Proximal phalanx of great toe 14 Distal phalanx of great toe 15 Proximal phalanx of second toe 16 Middle phalanx of second toe 17 Distal phalanx of second toe Bones of the tarsus, the back part of the foot Talus Calcaneus Navicular bone Cuboid bone Medial, intermediate and lateral cuneiform bones Bones of the metatarsus, the forepart of the foot First to fifth metatarsal bones (numbered from the medial side) Bones of the toes or digits Phalanges -- a proximal and a distal phalanx for the great toe; proximal, middle and distal phalanges for the second to fifth toes Sesamoid bones Two always present in the tendons of flexor hallucis brevis Origin and meaning of some terms associated with the foot Tibia: Latin for a flute or pipe; the shin bone has a fanciful resemblance to this wind instrument. Fibula: Latin for a pin or skewer; the long thin bone of the leg. Adjective fibular or peroneal, which is from the Greek for pin. Tarsus: Greek for a wicker frame; the basic framework for the back of the foot. Metatarsus: Greek for beyond the tarsus; the forepart of the foot. Talus (astragalus): Latin (Greek) for one of a set of dice; viewed from above the main part of the talus has a rather square appearance. -

Multiplanar CT Analysis of Fifth Metatarsal Morphology
FAIXXX10.1177/1071100715623041Foot & Ankle InternationalDeSandis et al 623041research-article2015 Article Foot & Ankle International® 2016, Vol. 37(5) 528 –536 Multiplanar CT Analysis of Fifth Metatarsal © The Author(s) 2015 Reprints and permissions: sagepub.com/journalsPermissions.nav Morphology: Implications for Operative DOI: 10.1177/1071100715623041 Management of Zone II Fractures fai.sagepub.com Bridget DeSandis, BA1, Conor Murphy, MD2, Andrew Rosenbaum, MD1, Matthew Levitsky, BA1, Quinn O’Malley1, Gabrielle Konin, MD1, and Mark Drakos, MD1 Abstract Background: Percutaneous internal fixation is currently the method of choice treating proximal zone II fifth metatarsal fractures. Complications have been reported due to poor screw placement and inadequate screw sizing. The purpose of this study was to define the morphology of the fifth metatarsal to help guide surgeons in selecting the appropriate screw size preoperatively. Methods: Multiplanar analysis of fifth metatarsal morphology was completed using computed tomographic (CT) scans from 241 patients. Specific parameters were analyzed and defined in anteroposterior (AP), lateral, and oblique views including metatarsal length, distance from the base to apex of curvature, apex medullary canal width, apex height, and fifth metatarsal angle. Results: The average metatarsal length in the AP view was 71.4 ± 6.1 mm and in the lateral view 70.4 ± 6.0 mm, with 95% of patients having lengths between 59.3 and 83.5 mm and 58.4 and 82.4 mm, respectively. The average canal width at the apex of curvature was 4.1 ± 0.9 mm in the AP view and 5.3 ± 1.1 mm in the lateral view, with 95% of patients having widths between 2.2 and 5.9 mm and 3.2 and 7.5 mm, respectively. -

Rethinking the Evolution of the Human Foot: Insights from Experimental Research Nicholas B
© 2018. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2018) 221, jeb174425. doi:10.1242/jeb.174425 REVIEW Rethinking the evolution of the human foot: insights from experimental research Nicholas B. Holowka* and Daniel E. Lieberman* ABSTRACT presumably owing to their lack of arches and mobile midfoot joints Adaptive explanations for modern human foot anatomy have long for enhanced prehensility in arboreal locomotion (see Glossary; fascinated evolutionary biologists because of the dramatic differences Fig. 1B) (DeSilva, 2010; Elftman and Manter, 1935a). Other studies between our feet and those of our closest living relatives, the great have documented how great apes use their long toes, opposable apes. Morphological features, including hallucal opposability, toe halluces and mobile ankles for grasping arboreal supports (DeSilva, length and the longitudinal arch, have traditionally been used to 2009; Holowka et al., 2017a; Morton, 1924). These observations dichotomize human and great ape feet as being adapted for bipedal underlie what has become a consensus model of human foot walking and arboreal locomotion, respectively. However, recent evolution: that selection for bipedal walking came at the expense of biomechanical models of human foot function and experimental arboreal locomotor capabilities, resulting in a dichotomy between investigations of great ape locomotion have undermined this simple human and great ape foot anatomy and function. According to this dichotomy. Here, we review this research, focusing on the way of thinking, anatomical features of the foot characteristic of biomechanics of foot strike, push-off and elastic energy storage in great apes are assumed to represent adaptations for arboreal the foot, and show that humans and great apes share some behavior, and those unique to humans are assumed to be related underappreciated, surprising similarities in foot function, such as to bipedal walking. -

5Th Metatarsal Fracture
FIFTH METATARSAL FRACTURES Todd Gothelf MD (USA), FRACS, FAAOS, Dip. ABOS Foot, Ankle, Shoulder Surgeon Orthopaedic You have been diagnosed with a fracture of the fifth metatarsal bone. Surgeons This tyPe of fracture usually occurs when the ankle suddenly rolls inward. When the ankle rolls, a tendon that is attached to the fifth metatarsal bone is J. Goldberg stretched. Because the bone is weaker than the tendon, the bone cracks first. A. Turnbull R. Pattinson A. Loefler All bones heal in a different way when they break. This is esPecially true J. Negrine of the fifth metatarsal bone. In addition, the blood suPPly varies to different I. PoPoff areas, making it a lot harder for some fractures to heal without helP. Below are D. Sher descriPtions of the main Patterns of fractures of the fifth metatarsal fractures T. Gothelf and treatments for each. Sports Physicians FIFTH METATARSAL AVULSION FRACTURE J. Best This fracture Pattern occurs at the tiP of the bone (figure 1). These M. Cusi fractures have a very high rate of healing and require little Protection. Weight P. Annett on the foot is allowed as soon as the Patient is comfortable. While crutches may helP initially, walking without them is allowed. I Prefer to Place Patients in a walking boot, as it allows for more comfortable walking and Protects the foot from further injury. RICE treatment is initiated. Pain should be exPected to diminish over the first four weeks, but may not comPletely go away for several months. Follow-uP radiographs are not necessary if the Pain resolves as exPected. -

Common Stress Fractures BRENT W
COVER ARTICLE PRACTICAL THERAPEUTICS Common Stress Fractures BRENT W. SANDERLIN, LCDR, MC, USNR, Naval Branch Medical Clinic, Fort Worth, Texas ROBERT F. RASPA, CAPT, MC, USN, Naval Hospital Jacksonville, Jacksonville, Florida Lower extremity stress fractures are common injuries most often associated with partic- ipation in sports involving running, jumping, or repetitive stress. The initial diagnosis can be made by identifying localized bone pain that increases with weight bearing or repet- itive use. Plain film radiographs are frequently unrevealing. Confirmation of a stress frac- ture is best made using triple phase nuclear medicine bone scan or magnetic resonance imaging. Prevention of stress fractures is most effectively accomplished by increasing the level of exercise slowly, adequately warming up and stretching before exercise, and using cushioned insoles and appropriate footwear. Treatment involves rest of the injured bone, followed by a gradual return to the sport once free of pain. Recent evidence sup- ports the use of air splinting to reduce pain and decrease the time until return to full par- ticipation or intensity of exercise. (Am Fam Physician 2003;68:1527-32. Copyright© 2003 American Academy of Family Physicians) tress fractures are among the involving repetitive use of the arms, such most common sports injuries as baseball or tennis. Stress fractures of and are frequently managed the ribs occur in sports such as rowing. by family physicians. A stress Upper extremity and rib stress fractures fracture should be suspected in are far less common than lower extremity Sany patient presenting with localized stress fractures.1 bone or periosteal pain, especially if he or she recently started an exercise program Etiology and Pathophysiology or increased the intensity of exercise. -

ASSESSMENT of FIFTH METATARSAL ETIOLOGY Daniel Reed Clemson University, [email protected]
Clemson University TigerPrints All Theses Theses 7-2008 ASSESSMENT OF FIFTH METATARSAL ETIOLOGY Daniel Reed Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_theses Part of the Biomedical Engineering and Bioengineering Commons Recommended Citation Reed, Daniel, "ASSESSMENT OF FIFTH METATARSAL ETIOLOGY" (2008). All Theses. 428. https://tigerprints.clemson.edu/all_theses/428 This Thesis is brought to you for free and open access by the Theses at TigerPrints. It has been accepted for inclusion in All Theses by an authorized administrator of TigerPrints. For more information, please contact [email protected]. ASSESSMENT OF FIFTH METATARSAL FRACTURE ETIOLOGY A Thesis Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirement for the Degree Master of Science Bioengineering by Daniel Reed August 2008 Accepted by: Dr. Martine Laberge, Committee Chair Dr. Lisa Benson Dr. Larry Bowman MD ABSTRACT The fifth metatarsal “Jones Fracture” is a fracture that occurs 3.5cm distal to the tuberosity. It is an injury that is common in athletes, especially those who participate in sports with a lot of lateral movement. The Jones Fracture is known for its difficulty to heal due to non-union and re-fracture. There has been much research recently regarding in-shoe pressure distributions and their relation to shoe type, movement, and shoe surface interaction. However, only the forces along the bottom of the foot have been investigated. Literature and the direction of fracture seem to implicate a force on the lateral portion of the foot is the cause of the fracture though the exact causal forces are still largely unknown. -

Metric and Non Metric Characteristics of Human Forefoot: a Radiological Study in Egyptian Population
Australian Journal of Basic and Applied Sciences 2019 February; 13(2): pages 46-54 DOI: 10.22587/ajbas.2019.13.2.6 Original paper AENSI Publications Journal home page: www.ajbasweb.com Metric and Non metric characteristics of human forefoot: A radiological study in Egyptian population Samah Mohammed, Mahmoud Abozaid and Fatma Elzahraa, Fouad Abd Elbaky Department of Anatomy, Faculty of medicine, Minia University, Minia, Egypt. Correspondence Author: Samah Mohammed, Department of Anatomy, Faculty of medicine, Minia University, Minia, Egypt. Received date: 1 January 2019, Accepted date: 15 January 2018, Online date: 28 February 2019 Copyright: © 2019 Samah Mohammed et al, This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Human forefoot is composed of metatarsal bones and phalanges. Their morphology and dimensions are important for proper bipedal locomotor function. Structural defects affecting forefoot bones result in foot dysfunction. The aim of the present study is to describe the morphology of the bones of forefoot and to measure their lengths and widths. A digital radiographic study was conducted on right and left feet of 100 healthy individuals (50 males and 50 females) above 21 years old. Inspection of the forefoot bones using radiographs was done to describe general shapes of bones. The lengths and widths of metatarsal bone and phalanges were measured using Digital Imaging and Communications in Medicine (DICOM) format. Mean and standard deviations were obtained for all measurements of each side of both sexes. -

A Morphometric and Morphological Study on Dry Adult Cuboid Bones
Review Article Clinician’s corner Images in Medicine Experimental Research Case Report Miscellaneous Letter to Editor DOI: 10.7860/JCDR/2021/47259.14447 Original Article Postgraduate Education A Morphometric and Morphological Case Series Study on Dry Adult Cuboid Bones Research Protocol Anatomy Section Short Communication SREYA MOITRA ABSTRACT software, version 19. Students t-test was applied to find the Introduction: Precise biometric data of cuboid and difference between the mean values of the parameters. calcaneocuboid joint are not discussed very distinctly in the text Results: Mean medial length of cuboid was 33.41 mm, lateral books of Anatomy. A better knowledge of the joint surfaces of length was 19.73 mm, height was 26.17 mm, length index cuboid and biometric data would generate a three dimensional was 169.33, vertical and transverse diameters of calcaneal modeling of the calcaneocuboid joint and would help in the articular facet were 24.24 mm and 16.45 mm respectively, vertical and transverse diameters of metatarsal articular facet management of Cuboid Syndrome. were 21.32 mm and 13.85 mm respectively, depth of peroneal Aim: To study about morphological and morphometric analysis groove was 0.63mm. Concavo-convex facet with posteromedial in adult dry cuboid bone. projection and oval or reniform in shape (Type 1A) was the most Materials and Methods: This study was conducted in the common calcaneal articular facet and convex pattern was the Department of Anatomy of a Medical College using 60 dry most common metatarsal articular facet of cuboid. cuboid bones from museum. Each bone was observed for its Conclusion: Morphological characterisation of articular facet morphometric analysis as well as its pattern of calcaneal and of cuboid and its morphometric analysis help to understand the metatarsal articular facets. -

Case Report: Atypical Metatarsal Fracture in a Patient on Long- Term Bisphosphonate Therapy
November 2019. Volume 6. Number 4 Case Report: ATypical Metatarsal Fracture in a Patient on Long- Term Bisphosphonate Therapy Bijan Valiollahi1 , Mostafa Salehpour1 , Hamidreza Bashari1 , Shoeib Majdi1 , Mehdi Mohammadpour1 * 1. Bone and Joint Reconstruction Research Center, Shafa Orthopedic Hospital, Iran University of Medical Sciences, Tehran, Iran. Use your device to scan and read the article online Citation Valiollahi B, Salehpour M, Bashari H, Majdi Sh, Mohammadpour M. ATypical Metatarsal Fracture in a Patient on Long-Term Bisphosphonate Therapy. Journal of Research in Orthopedic Science. 2019; 6(4):25-30. http://dx.doi.org/10.32598/ JROSJ.6.4.67 : http://dx.doi.org/10.32598/JROSJ.6.4.67 A B S T R A C T Bisphosphonates, more particularly alendronate, are a popular category of drugs in the treatment Article info: of postmenopausal and corticosteroid-induced osteoporosis. The present study contends that the Received: 13 May 2019 long-term consumption of bisphosphonates causes not only subtrochanteric and femoral shaft Revised: 27 May 2019 fractures but also pathological fractures at other musculoskeletal sites. This report presents a Accepted: 16 Sep 2019 rare case of alendronate-induced pathological metatarsal fracture in a 59-year-old female with a Available Online: 01 Nov 2019 history of cuboid fracture following a twisting with abnormal Bone Mineral Density (BMD) (T score: −3.5; lumbar spine and −2.6; proximal femur). Keywords: Alendronate, Pathologic Fracture, Metatarsal 1. Introduction ally, drugs such as bisphosphonates contribute to devel- oping bone fractures. tress fractures occur as a result of repetitive loading and unloading of a bone [1]. In- Bisphosphonates are preferred drugs in postmenopaus- creased strain or frequency of compression al and corticosteroid-induced osteoporosis [6]. -

Broken Foot (Fractured Metatarsal)
www.healthinfo.org.nz Broken foot (fractured metatarsal) Your metatarsal bones are the long, straight bones in the middle of your foot. If your metatarsals are fractured, it means they are broken. Metatarsal bones often break when a heavy object falls on to your foot. The outside bone can also break because of a twisting injury at the ankle or by kicking a heavy object. Runners can develop stress fractures in these bones, caused by repeated force on their feet. Treating broken metatarsals A broken metatarsal can be treated in different ways. If your bone is broken in a good position but is stable, you may be put into a plaster of Paris cast to support your foot and stop the broken bone from moving while it heals. You will learn how to use crutches or a frame to get around. Your doctor or nurse will also tell you how much weight you can put through your injured foot. You will have a follow-up appointment at the Orthopaedics Outpatient Clinic one to two weeks after your injury to check how well you are healing. This will include an X-ray. If the metatarsal on the outside of your foot has a small break (called an avulsion fracture), it may be treated with a Tubigrip bandage and crutches to help you walk. You might need an operation to move the bones in to the right position and fix them in place. This will happen if the broken bones are not in a good position or are unstable, or if you have also dislocated the joint of your bone. -

Stress Fractures in the Foot and Ankle of Athletes Fratura Por Estresse No Pé E Tornozelo De Atletas Authors: Asano LYJ, Duarte Jr
GUIDELINES IN FOCUS ASANO LYJ ET al. Stress fractures in the foot and ankle of athletes FRATURA POR ESTRESSE NO PÉ E TORNOZELO DE ATLETAS Authors: Asano LYJ, Duarte Jr. A, Silva APS http://dx.doi.org/10.1590/1806-9282.60.06.006 The Guidelines Project, an initiative of the Brazilian Medical Association, aims to combine information from the medical field in order to standar- dize procedures to assist the reasoning and decision-making of doctors. The information provided through this project must be assessed and criticized by the physician responsible for the conduct that will be adopted, de- pending on the conditions and the clinical status of each patient. DESCRIPTION OF THE EVIDENCE COLLECTION INTRODUCTION METHOD Stress fractures were described for the first time in 1855 To develop this guideline, the Medline electronic databa- by Breihaupt among soldiers reporting plantar pain and se (1966 to 2012) was consulted via PubMed, as a primary edema following long marches.1 For athletes, the first cli- base. The search for evidence came from actual clinical nical description was given by Devas in 1958, based so- scenarios and used keywords (MeSH terms) grouped in lely on the results of simple X-rays.2 Stress injuries are the following syntax: “Stress fractures”, “Foot”, “Ankle”, common among athletes and military recruits, accoun- “Athletes”, “Professional”, “Military recruit”, “Immobili- ting for approximately 10% of all orthopedic injuries.3 zation”, “Physiotherapy”, “Rest”, “Rehabilitation”, “Con- It is defined as a solution for partial or complete con- ventional treatment”, “Surgery treatment”. The articles tinuity of a bone as a result of excessive or repeated loads, were selected by orthopedic specialists after critical eva- at submaximal intensity, resulting in greater reabsorp- luation of the strength of scientific evidence, and publi- tion faced with an insufficient formation of bone tissue.1 cations of greatest strength were used for recommenda- Although stress fractures may affect all types of bone tion. -

A Biomechanical Model of the Foot
0031-9290861300 + 00 C 1986 Pcrgamon Journals Ltd. A BIOMECHANICAL MODEL OF THE FOOT ERICPAULSALATHL JR Eastern Scientific and Education Foundation, 32 North West Street, Allentown. PA 18102.U.S.A. GEORGEA.ARANGIO Eastern Scientific and Education Foundation and Lehigh Valley Hospital Center, Allentown, PA 18103, U.S.A. and ERIC P. SALATH~ Department of Mathematics, Lehigh University, Bethlehem, PA 18015, U.S.A. Abstract-The foot is modeled as a statically indeterminate structure supporting its load at the heads of the five metatarsais and the tuberosity of the calcaneous. The distribution of support is determined through an analysis of the deformations caused in the structure as a result of the forces at these locations. The analysis includes the effect of the plantar aponeurosis and takes into account the deformation of the metatarsals and bending of the joints. A parametric study is presented to illustrate the behavior of the solution under a broad range of conditions. INTRODUCIION (1954) suggested that the interplay between truss action, dominant in the heel up position when the Knowledge of the stresses that occur throughout windlass eRect increases tension in the plantar ap- normal and abnormal feet under various loading oneurosis, and beam action,significant when the foot is conditions is of fundamental importance in under- flat and the toes are straight, results in large variation standing the physiology and pathophysiology of the of bending stresses on the metatarsals. He supported foot, and can provide important information for the thisconcept through a simple but clever experiment, in prevention and treatment of injuries. The foot is an which he mounted mirrors at the base and head of each arch shaped structure carrying loads applied to the metatarsal and measured the amount of bending by talus by the tibia and fibula.