RAD Sequencing Sheds New Light on the Genetic Structure and Local Adaptation of European Scallops and Resolves Their Demographic Histories David L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Os Nomes Galegos Dos Moluscos
A Chave Os nomes galegos dos moluscos 2017 Citación recomendada / Recommended citation: A Chave (2017): Nomes galegos dos moluscos recomendados pola Chave. http://www.achave.gal/wp-content/uploads/achave_osnomesgalegosdos_moluscos.pdf 1 Notas introdutorias O que contén este documento Neste documento fornécense denominacións para as especies de moluscos galegos (e) ou europeos, e tamén para algunhas das especies exóticas máis coñecidas (xeralmente no ámbito divulgativo, por causa do seu interese científico ou económico, ou por seren moi comúns noutras áreas xeográficas). En total, achéganse nomes galegos para 534 especies de moluscos. A estrutura En primeiro lugar preséntase unha clasificación taxonómica que considera as clases, ordes, superfamilias e familias de moluscos. Aquí apúntase, de maneira xeral, os nomes dos moluscos que hai en cada familia. A seguir vén o corpo do documento, onde se indica, especie por especie, alén do nome científico, os nomes galegos e ingleses de cada molusco (nalgún caso, tamén, o nome xenérico para un grupo deles). Ao final inclúese unha listaxe de referencias bibliográficas que foron utilizadas para a elaboración do presente documento. Nalgunhas desas referencias recolléronse ou propuxéronse nomes galegos para os moluscos, quer xenéricos quer específicos. Outras referencias achegan nomes para os moluscos noutras linguas, que tamén foron tidos en conta. Alén diso, inclúense algunhas fontes básicas a respecto da metodoloxía e dos criterios terminolóxicos empregados. 2 Tratamento terminolóxico De modo moi resumido, traballouse nas seguintes liñas e cos seguintes criterios: En primeiro lugar, aprofundouse no acervo lingüístico galego. A respecto dos nomes dos moluscos, a lingua galega é riquísima e dispomos dunha chea de nomes, tanto específicos (que designan un único animal) como xenéricos (que designan varios animais parecidos). -

DEEP SEA LEBANON RESULTS of the 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project
DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project March 2018 DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project Citation: Aguilar, R., García, S., Perry, A.L., Alvarez, H., Blanco, J., Bitar, G. 2018. 2016 Deep-sea Lebanon Expedition: Exploring Submarine Canyons. Oceana, Madrid. 94 p. DOI: 10.31230/osf.io/34cb9 Based on an official request from Lebanon’s Ministry of Environment back in 2013, Oceana has planned and carried out an expedition to survey Lebanese deep-sea canyons and escarpments. Cover: Cerianthus membranaceus © OCEANA All photos are © OCEANA Index 06 Introduction 11 Methods 16 Results 44 Areas 12 Rov surveys 16 Habitat types 44 Tarablus/Batroun 14 Infaunal surveys 16 Coralligenous habitat 44 Jounieh 14 Oceanographic and rhodolith/maërl 45 St. George beds measurements 46 Beirut 19 Sandy bottoms 15 Data analyses 46 Sayniq 15 Collaborations 20 Sandy-muddy bottoms 20 Rocky bottoms 22 Canyon heads 22 Bathyal muds 24 Species 27 Fishes 29 Crustaceans 30 Echinoderms 31 Cnidarians 36 Sponges 38 Molluscs 40 Bryozoans 40 Brachiopods 42 Tunicates 42 Annelids 42 Foraminifera 42 Algae | Deep sea Lebanon OCEANA 47 Human 50 Discussion and 68 Annex 1 85 Annex 2 impacts conclusions 68 Table A1. List of 85 Methodology for 47 Marine litter 51 Main expedition species identified assesing relative 49 Fisheries findings 84 Table A2. List conservation interest of 49 Other observations 52 Key community of threatened types and their species identified survey areas ecological importanc 84 Figure A1. -

On the Cephalopod Phosphagen by Ernest Baldwin, B.A
222 ON THE CEPHALOPOD PHOSPHAGEN BY ERNEST BALDWIN, B.A. (From the Biochemical Laboratory, Cambridge, and the Marine Biological Station, Tamaris, Var, France.) (Received 8th November, 1932.) (With Four Text-figures.) INTRODUCTION. THE comparative researches of Eggleton & Eggleton(s) on the distribution of phosphagen made it clear that while creatine phosphate is very widely distributed amongst the vertebrates, it is not present in the invertebrates. Shortly afterwards, a new phosphagenic substance was isolated from crab muscle by Meyerhof & Lohmann(n, 12) and shown to be arginine phosphate, while the later work of Lundsgaard (9) has made it certain that this compound plays in these tissues a part exactly analogous to that played by the creatine compound in vertebrate muscles. Later, Meyerhof (10) examined a number of invertebrates, representative of several phyla, and came to the conclusion that arginine phosphate is present in Holothuria, Pecten and Sipunculus. Cephalopod muscle contained no phosphagen. The case of the cephalopods was further examined by Needham, Needham, Baldwin & Yudkin (13), who not only found that the muscles of Sepia and of Octopus do contain phosphagen, but were also able to investigate its ontogeny in the former (14). There seemed no reason to think that the compound present was any- thing other than the arginine compound (8). Ackermann, Holtz & Kutscherw claim to have isolated the copper nitrate salt of arginine from extracts of the cephalopod Eledone moschata, while Okuda(is) has made a similar claim in the case of Loligo breekert, whereas Iseki (7) has been able to isolate no arginine from extracts of Octopus, finding in its place a compound which he isolated in the form of its picrate, and which he thinks may be a methyl agmatine. -

The Shell As a Symbolic Design Motif
THE SHELL AS A SYMBOLIC DESIGN MOTIF: RELIGIOUS SIGNIFICANCE AND USE IN SELECTED AREAS OF THE MISSION SAN XAVIER DEL BAC TUCSON, ARIZONA By LINDA ANNE TARALDSON Bachelor of Science University of Arizona Tucson, Arizona 1964 Submitted to the faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE May, 1968 ',' ,; OKLAHOMA STATE UNIVERSflY LIBRARY OCT ~ij 1968 THE SHELL AS A SYMBOLIC DESIGN MOTIF:,, ... _ RELIGIOUS SIGNIFICANCE AND USE IN SELECTED AREAS OF THE MISSION SAN XAVIER DEL BAC TUCSON, ARIZONA Thesis Approved: Dean of the Graduate College 688808 ii PREFACE The creative Interior Designer _needs to have a thorough knowledge and understanding of history and a skill in correlating authentic de sl-gns of the past with the present. Sensitivity to the art and designs of the past aids the Interior Designer in adapting them into the crea-_ tion of the contemporary interior, Des.igns of the past can have an integral relationship with contemporary design, Successful designs are those which have survived and have transcended time, Thus, the Interior Designer needs to know the background of a design, the original use of a design, and the period to which a design belongs in order to success fully adapt the design to the contemporary creation of beautyo This study of the shell as a symbolic design motif began with a profound interest in history, a deep love for a serene desert mission. and a probing curiosity concerning an outstanding design used in con -

Heavy Metals in the Antarctic Scallop Adam Ussium Colbecki
MARINE ECOLOGY PROGRESS SERIES Vol. 67: 27-33, 1990 Published September 20 Mar. Ecol. Prog. Ser. l Heavy metals in the Antarctic scallop Adam ussium colbecki Dipartirnento di Biologia Anirnale, Universita di Modena, via Universita 4,1-41100 Modena, Italy Dipartirnento di Biomedicina Sperimentale Infettiva e Pubblica, Universita di Pisa. via Volta 4.1-56100 Pisa, Italy ABSTRACT: Cu, Fe, Cr. Cd, Mn and Zn concentrations were determined in different organs of the Antarctic scallop Adarnussium colbecki (Smith) and compared with those found in Pecten jacobaeus L., a scallop of temperate waters, and wlth literature values for other Pectinidae. The digestive gland of A. colbeck, was the target organ for Cu, Fe, Cr and Cd, whereas Mn and Zn were found mainly in the kidney. Cd concentration in the digestive gland of A. colbecki was higher than that in the same organ of P. jacobaeus, indicating a marked ability of the Antarctic scallop to concentrate this metal. However, in A. colbecki renal concentrations of both Mn and Zn were considerably lower than those measured in P. lacobaeus and other Pectinidae, and may be related to the scarcity of concretions observed in its kidney. INTRODUCTION Pecten lacobaeus L., a hermaphroditic species of temperate waters, was used for comparison between Better insight into the ecology of the Antarctic is Adarnussiurn colbecki and other non-Antarctic scallops. today of great importance considering the increasing Data on heavy metal levels in other species of Pectinidae interest shown in the resources of this continent. Col- were also used for comparison with A. colbecki. lecting new environmental data will serve as the baseline for evaluating future environmental impact of pollutants in this remote area. -

Bivalve Mollusc Exploitation in Mediterranean Coastal Communities: an Historical Approach
Journal of Biological Research-Thessaloniki 12: 00 – 00, 2009 J. Biol. Res.-Thessalon. is available online at http://www.jbr.gr Indexed in: WoS (Web of Science, ISI Thomson), SCOPUS, CAS (Chemical Abstracts Service) and DOAJ (Directory of Open Access Journals) Bivalve mollusc exploitation in Mediterranean coastal communities: an historical approach ELENI VOULTSIADOU1*, DROSOS KOUTSOUBAS2 and MARIA ACHPARAKI1 1 Department of Zoology, School of Biology, Aristotle University of Thessaloniki, 541 24 Thessaloniki, Greece 2 Department of Marine Sciences, School of Environment, University of the Aegean, 81100 Mytilene, Greece Received: 3 July 2009 Accepted after revision: 14 September 2009 The aim of this work was to survey the early history of bivalve mollusc exploitation and con- sumption in the Mediterranean coastal areas as recorded in the classical works of Greek antiq- uity. All bivalve species mentioned in the classical texts were identified on the basis of modern taxonomy. The study of the works by Aristotle, Hippocrates, Xenocrates, Galen, Dioscorides and Athenaeus showed that out of the 35 exploited marine invertebrates recorded in the texts, 20 were molluscs, among which 11 bivalve names were included. These data examined under the light of recent information on bivalve exploitation showed that the diet of ancient Greeks in- cluded the same bivalve species consumed nowadays in the coastal areas of the Mediterranean. The habitats of the exploited bivalves and consequently their fishing areas were well known and recorded in the classical texts. Information on the morphology and various aspects of the biolo- gy of certain edible species was given mostly in Aristotle’s zoological works, while Xenocrates and Athenaeus presented instructions and recipes on how bivalves were cooked and served. -

Using Bivalve Chronologies for Quantifying Environmental Drivers in a Semi-Enclosed Temperate Sea Received: 7 December 2017 M
www.nature.com/scientificreports OPEN Using bivalve chronologies for quantifying environmental drivers in a semi-enclosed temperate sea Received: 7 December 2017 M. Peharda 1, I. Vilibić1, B. A. Black2, K. Markulin 1, N. Dunić1, T. Džoić1, H. Mihanović1, Accepted: 20 March 2018 M. Gačić3, S. Puljas4 & R. Waldman5 Published: xx xx xxxx Annual growth increments formed in bivalve shells are increasingly used as proxies of environmental variability and change in marine ecosystems, especially at higher latitudes. Here, we document that well-replicated and exactly dated chronologies can also be developed to capture oceanographic processes in temperate and semi-enclosed seas, such as the Mediterranean. A chronology is constructed for Glycymeris pilosa from a shallow embayment of the northern Adriatic and extends from 1979 to 2016. The chronology signifcantly (p < 0.05) and positively correlates to winter sea surface temperatures, but negatively correlates to summer temperatures, which suggests that extreme winter lows and extreme summer highs may be limiting to growth. However, the strongest and most consistent relationships are negative correlations with an index of the Adriatic-Ionian Bimodal Oscillating System (BiOS) for which positive values indicate the infow of the ultraoligotrophic Eastern Mediterranean waters to the Adriatic. In contrast, the substantial freshwater fows that discharge into the Adriatic do not correlate to the bivalve chronology, emphasizing the importance of remote oceanographic processes to growth at this highly coastal site. Overall, this study underscores the potential of bivalve chronologies to capture biologically relevant, local- to regional-scale patterns of ocean circulation in mid-latitude, temperate systems. A signifcant limitation in establishing the role of climate variability on ecological functioning in marine eco- systems is the lack of long-term observational records, particularly for biological phenomena, the lengths of which rarely exceed thirty years1. -

The Evolution of Extreme Longevity in Modern and Fossil Bivalves
Syracuse University SURFACE Dissertations - ALL SURFACE August 2016 The evolution of extreme longevity in modern and fossil bivalves David Kelton Moss Syracuse University Follow this and additional works at: https://surface.syr.edu/etd Part of the Physical Sciences and Mathematics Commons Recommended Citation Moss, David Kelton, "The evolution of extreme longevity in modern and fossil bivalves" (2016). Dissertations - ALL. 662. https://surface.syr.edu/etd/662 This Dissertation is brought to you for free and open access by the SURFACE at SURFACE. It has been accepted for inclusion in Dissertations - ALL by an authorized administrator of SURFACE. For more information, please contact [email protected]. Abstract: The factors involved in promoting long life are extremely intriguing from a human perspective. In part by confronting our own mortality, we have a desire to understand why some organisms live for centuries and others only a matter of days or weeks. What are the factors involved in promoting long life? Not only are questions of lifespan significant from a human perspective, but they are also important from a paleontological one. Most studies of evolution in the fossil record examine changes in the size and the shape of organisms through time. Size and shape are in part a function of life history parameters like lifespan and growth rate, but so far little work has been done on either in the fossil record. The shells of bivavled mollusks may provide an avenue to do just that. Bivalves, much like trees, record their size at each year of life in their shells. In other words, bivalve shells record not only lifespan, but also growth rate. -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste -

Lead, Mercury and Cadmium Levels in Edible Marine Molluscs and Echinoderms from the Veneto Region (North-Western Adriatic Sea – Italy)
Accepted Manuscript Lead, mercury and cadmium levels in edible marine molluscs and echinoderms from the Veneto Region (north-western Adriatic Sea – Italy) Laura Bille, Giovanni Binato, Veronica Cappa, Marica Toson, Manuela Dalla Pozza, Giuseppe Arcangeli, Antonia Ricci, Roberto Angeletti, Roberto Piro PII: S0956-7135(14)00524-6 DOI: 10.1016/j.foodcont.2014.09.018 Reference: JFCO 4067 To appear in: Food Control Received Date: 14 March 2014 Revised Date: 12 September 2014 Accepted Date: 15 September 2014 Please cite this article as: Bille L., Binato G., Cappa V., Toson M., Pozza M.D., Arcangeli G., Ricci A., Angeletti R. & Piro R., Lead, mercury and cadmium levels in edible marine molluscs and echinoderms from the Veneto Region (north-western Adriatic Sea – Italy), Food Control (2014), doi: 10.1016/ j.foodcont.2014.09.018. This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. ACCEPTED MANUSCRIPT 1 Lead, mercury and cadmium levels in edible marine molluscs and echinoderms 2 from the Veneto Region (north-western Adriatic Sea – Italy) 3 Laura Bille a * , Giovanni Binato a, Veronica Cappa a, Marica Toson a, Manuela Dalla 4 Pozza a, Giuseppe Arcangeli a, Antonia Riccia, Roberto Angeletti a, Roberto Piro a. -
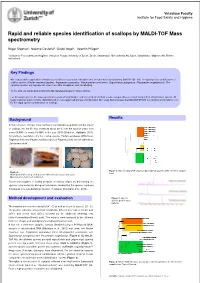
Rapid and Reliable Species Identification of Scallops by MALDI-TOF Mass Spectrometry
Vetsuisse Faculty Institute for Food Safety and Hygiene Rapid and reliable species identification of scallops by MALDI-TOF Mass spectrometry Roger Stephan 1, Noémie Oesterlé 2, Guido Vogel 3, Valentin Pflüger 3 1Institute for Food Safety and Hygiene, Vetsuisse Faculty University of Zurich, Zurich, Switzerland; 2Bell Schweiz AG, Basel, Switzerland; 3Mabritec AG, Riehen, Switzerland Key Findings We evaluated the application of matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) for rapid species identification of scallop species ( Pecten maximus /jacobei , Argopecten purpuratus , Mizuhopecten yessoensis , Zygochlamys patagonica , Placopecten magellanicus ). The selected species are important in view of possible deceptions and mislabelling. To this end, we developed a reference MS database library for these species. For the target species the mass spectrometry-based identification scheme yielded identical results compared to a second independent identification system. All target samples were correctly identified and no non-target sample was misidentified. Our study demonstrates that MALDI-TOF-MS is a reliable and powerful tool for the rapid species identification of scallops. Results Background In the last years scallops have reached a considerable popularity and the import of scallops into the EU has increased about 20 % over the last five years from some 50.000 t to nearly 63.000 t in the year 2010 (Globefish, Highlights 2011 ). According to legislation only the scallop species Pecten jacobaeus (Mittelmeer- Pilgermuschel) and Pecten maximus (Grosse Pilgermuschel) can be labelled as „Jakobsmuscheln“. A) B) Figure 2: Mitochondrial DNA sequence based dendrogramm of the reference samples Figure 1: used. A) Characteristic scallop shell and shell with adductor muscle and corail . -

C:\Myfiles\Final Gear Workshop Report 2-20-02.Wpd
Northeast Fisheries Science Center Reference Document 02-01 Workshop on the Effects of Fishing Gear on Marine Habitats off the Northeastern United States October 23-25, 2001 Boston, Massachusetts by Northeast Region Essential Fish Habitat Steering Committee February 2002 Recent Issues in This Series: 01-03 Assessment of the Silver Hake Resource in the Northwest Atlantic in 2000. By J.K.T. Brodziak, E.M. Holmes, K.A. Sosebee, and R.K. Mayo. [A report of Northeast Regional Stock Assessment Workshop No. 32.] March 2001. 01-04 Report of the 32nd Northeast Regional Stock Assessment Workshop (32nd SAW): Public Review Workshop. [By the 32nd Northeast Regional Stock Assessment Workshop.] April 2001. 01-05 Report of the 32nd Northeast Regional Stock Assessment Workshop (32nd SAW): Stock Assessment Review Committee (SARC) Consensus Summary of Assessments. [By the 32nd Northeast Regional Stock Assessment Workshop.] April 2001. 01-06 Defining Triggers for Temporary Area Closures to Protect Right Whales from Entanglements: Issues and Op- tions. By P.J. Clapham and R.M. Pace, III. April 2001. 01-07 Proceedings of the 14th Canada-USA Scientific Discussions, January 22-25, 2001, MBL Conference Center, Woods Hole, Massachusetts. By S. Clark and R. O'Boyle, convenors. May 2001. 01-08 TRAC Advisory Report on Stock Status: A Report of the Fourth Meeting of the Transboundary Resources Assessment Committee (TRAC), St. Andrews Biological Station, St. Andrews, New Brunswick, April 17-20, 2001. [By the 4th Transboundary Resources Assessment Committee Meeting.] July 2001. 01-09 Results of a Field Collection of Biopsy Samples from Coastal Bottlenose Dolphin in the Mid-Atlantic.