Ankyrins, Spectrins, and Their Functional Patterning of Neurons
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Obituary "E" Index
Obituary "E" Index Copyright © 2004 - 2021 GRHS DISCLAIMER: GRHS cannot guarantee that should you purchase a copy of what you would expect to be an obituary from its obituary collection that you will receive an obituary per se. The obituary collection consists of such items as a) personal cards of information shared with GRHS by researchers, b) www.findagrave.com extractions, c) funeral home cards, d) newspaper death notices, and e) obituaries extracted from newspapers and other publications as well as funeral home web sites. Some obituaries are translations of obituaries published in German publications, although generally GRHS has copies of the German versions. These German versions would have to be ordered separately for they are kept in a separate file in the GRHS library. The list of names and dates contained herein is an alphabetical listing [by surname and given name] of the obituaries held at the Society's headquarters for the letter combination indicated. Each name is followed by the birth date in the first column and death date in the second. Dates may be extrapolated or provided from another source. Important note about UMLAUTS: Surnames in this index have been entered by our volunteers exactly as they appear in each obituary but the use of characters with umlauts in obits has been found to be inconsistant. For example the surname Büchele may be entered as Buchele or Bahmüller as Bahmueller. This is important because surnames with umlauted characters are placed in alphabetic order after regular characters so if you are just scrolling down this sorted list you may find the surname you are looking for in an unexpected place (i.e. -

Spectrin Binding Motifs Control Scribble Cortical Dynamics And
1 2 3 Spectrin binding motifs control Scribble cortical 4 dynamics and polarity function 5 6 Batiste Boëda and Sandrine Etienne Manneville 7 8 Institut Pasteur (CNRS URA 3691-INSERM), France 9 Correspondance to Sandrine Etienne-Manneville. Cell Polarity, Migration and Cancer Unit, 10 Institut Pasteur, 25 rue du Dr Roux, 75724 Paris cedex 15, France; Phone: +33 1 4438 9591; 11 FAX: +33 1 4568 8548. 12 e-mail: [email protected] 13 14 Competing interests statement: 15 The authors declare that no competing interests exist. 16 17 1 18 Abstract 19 The tumor suppressor protein Scribble (SCRIB) plays an evolutionary conserved role in 20 cell polarity. Despite being central for its function, the molecular basis of SCRIB 21 recruitment and stabilization at the cell cortex is poorly understood. Here we show that 22 SCRIB binds directly to the CH1 domain of spectrins, a molecular scaffold that 23 contributes to the cortical actin cytoskeleton and connects it to the plasma membrane. 24 We have identified a short evolutionary conserved peptide motif named SADH motif 25 (SCRIB ABLIMs DMTN Homology) which is necessary and sufficient to mediate protein 26 interaction with spectrins. The SADH domains contribute to SCRIB dynamics at the 27 cell cortex and SCRIB polarity function. Furthermore, mutations in SCRIB SADH 28 domains associated with spina bifida and cancer impact the stability of SCRIB at the 29 plasma membrane, suggesting that SADH domain alterations may participate in human 30 pathology. 31 32 33 34 35 36 37 2 38 Introduction 39 The protein SCRIB has been implicated in a staggering array of cellular processes 40 including polarity, migration, proliferation, differentiation, apoptosis, stemcell 41 maintenance, and vesicle trafficking [1]. -

Presidents' Circle
PRESIDENTS’ CIRCLE The donors celebrated below have contributed $1,500 or more to Support from the Presidents’ Circle has an immediate impact on the Annual Giving Program between July 1, 2015 and June 30, the lives of current students who rely on financial assistance to 2016, and represent the fiscal year 2016 Presidents’ Circle. achieve their educational goals. In short, Presidents’ Circle, you’re Presidents’ Circle members are provided with the opportunity to: the reason these students get to keep experiencing Saint Ben’s. This provide feedback, experience the impact of contributions and means your gift is valuable beyond measure. receive exclusive updates about the health and future of Saint Ben’s. Trustee Deceased Kapsner Society ($50,000 and above) Lois Schrantz Welshons Michael Contardo and Marilynn Olsen Anonymous Margie and John Wiehoff Mary Jo and Pete Conzemius Helga and Rick Bauerly Iris Cornelius and David Washington Shelly Bauerly Kopel and Scott Kopel Renner Society ($5,000 - $9,999) Theresa M. Coskran Patrick K. Edeburn Anonymous (2) Mark and Janet DeOrio Burton J. McGlynn Annette Atkins and Thomas Joyce Clara Dolan Guy and Barbara Schoenecker Rebecca and Christopher Coborn Karol and Larry Eckel Gregory and Stacy Schumacher Elizabeth L. Conrad Kathleen and Casey Eichler Megan and Joe Deignan Kris and Patrick Ellingsworth Westkaemper Society Peter and Kristin Diliberti Mark and Teresa Fleischhacker ($25,000 - $49,999) Terrance and Susan Dolan Agnes and Robert Flynn Harvey Bock Mary Dombovy and Michael Johnson Gregory and Julie Ann Frandsen Daniel and Mabel Coborn Kathleen and Terry Dooley Michael and Vera Fulks James and Catherine Denny Jeffrey Gau Kelly Ann and Mark Giura Virginia Ziebol Lyon Kathy and James Henderson Betty Beacom Hall Elizabeth Nilles Mary Dana Hinton and Robert Williams Laura and Brad Helferich Robert and Joyce Humboldt Annette P. -

Actinin-4 Reveals a Mechanism for Regulating Its F-Actin-Binding Affinity
Disease-associated mutant ␣-actinin-4 reveals a mechanism for regulating its F-actin-binding affinity Astrid Weins*, Johannes S. Schlondorff*, Fumihiko Nakamura†, Bradley M. Denker*, John H. Hartwig†, Thomas P. Stossel†, and Martin R. Pollak*‡ *Renal and †Translational Medicine Divisions, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA 02115 Edited by Thomas D. Pollard, Yale University, New Haven, CT, and approved August 23, 2007 (received for review March 16, 2007) ␣-Actinin-4 is a widely expressed protein that employs an actin- them cell motility, endocytosis, and adhesion (7–9). Cultured binding site with two calponin homology domains to crosslink podocytes deficient in one of the nonmuscle isotypes, ␣-actinin-4, actin filaments (F-actin) in a Ca2؉-sensitive manner in vitro.An exhibit defective substrate adhesion (10), which is consistent with inherited, late-onset form of kidney failure is caused by point the well documented localization of ␣-actinin at focal adhesion sites mutations in the ␣-actinin-4 actin-binding domain. Here we show (11), and is therefore probably responsible for the impaired trans- that ␣-actinin-4/F-actin aggregates, observed in vivo in podocytes lational locomotion of the ␣-actinin-4-deficient cells (7). of humans and mice with disease, likely form as a direct result of Five distinct point mutations in the ␣-actinin-4 head domain the increased actin-binding affinity of the protein. We document causing focal segmental glomerulosclerosis in affected humans all that exposure of a buried actin-binding site 1 in mutant ␣-actinin-4 mediate increased actin binding (12, 13). However, this effect has causes an increase in its actin-binding affinity, abolishes its Ca2؉ not yet been studied in detail, and the mechanism of disease has so regulation in vitro, and diverts its normal localization from actin far remained elusive. -

Current Understanding of the Role of Cytoskeletal Cross-Linkers in the Onset and Development of Cardiomyopathies
International Journal of Molecular Sciences Review Current Understanding of the Role of Cytoskeletal Cross-Linkers in the Onset and Development of Cardiomyopathies Ilaria Pecorari 1, Luisa Mestroni 2 and Orfeo Sbaizero 1,* 1 Department of Engineering and Architecture, University of Trieste, 34127 Trieste, Italy; [email protected] 2 University of Colorado Cardiovascular Institute, University of Colorado Anschutz Medical Campus, Aurora, CO 80045, USA; [email protected] * Correspondence: [email protected]; Tel.: +39-040-5583770 Received: 15 July 2020; Accepted: 10 August 2020; Published: 15 August 2020 Abstract: Cardiomyopathies affect individuals worldwide, without regard to age, sex and ethnicity and are associated with significant morbidity and mortality. Inherited cardiomyopathies account for a relevant part of these conditions. Although progresses have been made over the years, early diagnosis and curative therapies are still challenging. Understanding the events occurring in normal and diseased cardiac cells is crucial, as they are important determinants of overall heart function. Besides chemical and molecular events, there are also structural and mechanical phenomena that require to be investigated. Cell structure and mechanics largely depend from the cytoskeleton, which is composed by filamentous proteins that can be cross-linked via accessory proteins. Alpha-actinin 2 (ACTN2), filamin C (FLNC) and dystrophin are three major actin cross-linkers that extensively contribute to the regulation of cell structure and mechanics. Hereby, we review the current understanding of the roles played by ACTN2, FLNC and dystrophin in the onset and progress of inherited cardiomyopathies. With our work, we aim to set the stage for new approaches to study the cardiomyopathies, which might reveal new therapeutic targets and broaden the panel of genes to be screened. -
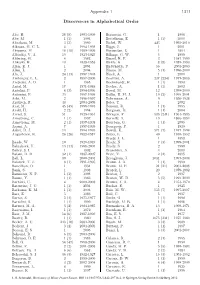
Appendix 1 1311 Discoverers in Alphabetical Order
Appendix 1 1311 Discoverers in Alphabetical Order Abe, H. 28 (8) 1993-1999 Bernstein, G. 1 1998 Abe, M. 1 (1) 1994 Bettelheim, E. 1 (1) 2000 Abraham, M. 3 (3) 1999 Bickel, W. 443 1995-2010 Aikman, G. C. L. 4 1994-1998 Biggs, J. 1 2001 Akiyama, M. 16 (10) 1989-1999 Bigourdan, G. 1 1894 Albitskij, V. A. 10 1923-1925 Billings, G. W. 6 1999 Aldering, G. 4 1982 Binzel, R. P. 3 1987-1990 Alikoski, H. 13 1938-1953 Birkle, K. 8 (8) 1989-1993 Allen, E. J. 1 2004 Birtwhistle, P. 56 2003-2009 Allen, L. 2 2004 Blasco, M. 5 (1) 1996-2000 Alu, J. 24 (13) 1987-1993 Block, A. 1 2000 Amburgey, L. L. 2 1997-2000 Boattini, A. 237 (224) 1977-2006 Andrews, A. D. 1 1965 Boehnhardt, H. 1 (1) 1993 Antal, M. 17 1971-1988 Boeker, A. 1 (1) 2002 Antolini, P. 4 (3) 1994-1996 Boeuf, M. 12 1998-2000 Antonini, P. 35 1997-1999 Boffin, H. M. J. 10 (2) 1999-2001 Aoki, M. 2 1996-1997 Bohrmann, A. 9 1936-1938 Apitzsch, R. 43 2004-2009 Boles, T. 1 2002 Arai, M. 45 (45) 1988-1991 Bonomi, R. 1 (1) 1995 Araki, H. 2 (2) 1994 Borgman, D. 1 (1) 2004 Arend, S. 51 1929-1961 B¨orngen, F. 535 (231) 1961-1995 Armstrong, C. 1 (1) 1997 Borrelly, A. 19 1866-1894 Armstrong, M. 2 (1) 1997-1998 Bourban, G. 1 (1) 2005 Asami, A. 7 1997-1999 Bourgeois, P. 1 1929 Asher, D. -

CH Domains Revisited
View metadata,FEBS Letters citation 431 and (1998) similar 134^137 papers at core.ac.uk broughtFEBS to you 20514 by CORE provided by Elsevier - Publisher Connector Minireview CH domains revisited Theresia Stradala, Wolfgang Kranewittera, Steven J. Winderb, Mario Gimonaa;* aInstitute of Molecular Biology, Austrian Academy of Sciences, Department of Cell Biology, Billrothstrasse 11, A-5020 Salzburg, Austria bInstitute of Cell and Molecular Biology, University of Edinburgh, May¢eld Road, Edinburgh EH9 3JR, UK Received 2 June 1998 responding to one CH domain [9^11]. Together, this led to the Abstract A sequence motif of about 100 amino acids, termed the `calponin homology domain' has been suggested to confer commonly accepted misconception that CH domains function actin binding to a variety of cytoskeletal and signalling as an autonomous F-actin binding domain, and the CH do- molecules. Here we analyse and compare the sequences of all main was installed as the prototype for a novel actin-binding calponin homology domain-containing proteins identified to date. module [3,4]. Closer inspection of CH domain sequences, We propose that single calponin homology domains do not confer however, reveals that this region does not overlap the estab- actin-binding per se and that the actin-binding motifs of cross- lished actin-binding domain in calponin, delineated in several linking proteins, which comprise two disparate calponin homol- independent studies [12^14]. Moreover, a second actin-target- ogy domains, represent a unique protein module. ing site was recently identi¢ed in the C-terminal tandem re- z 1998 Federation of European Biochemical Societies. peats of calponin [15], and it was further demonstrated that the single CH domain is neither su¤cient nor necessary for F- Key words: Calponin; Calponin homology domain; Actin binding actin binding of calponin and SM22 [16]. -

Honoring Life, Supporting Growth. MESSAGE from the EXECUTIVE DIRECTOR
2017 ANNUAL REPORT Honoring Life, Supporting Growth. MESSAGE FROM THE EXECUTIVE DIRECTOR Plymouth’s mission is about providing homes and comprehensive support to those who have survived without a safe place to live. What underpins the mission, however, is a new housing development at South King Street respect for the lives of the women and men who and Rainier Avenue, you’ll find that Plymouth is have been left by the wayside as our city grows growing swiftly, yet strategically, to bring even and social safety nets dwindle. more people out of homelessness and give them the support they need to thrive. Over my 25 years at Plymouth, our organization has nearly tripled the number of residents we Within the pages of this report, you’ll also learn serve. While we’ve grown a lot, the core of who about the life-changing work of Plymouth’s we are and why we’re here is the same. In fact, employees. Every day, our team lives our we are ever stronger in our mission. We let the mission. They support our residents through voices and needs of the people we serve—and challenges, celebrate every small success, and the neighborhoods we work in—guide us as we honor the dignity, uniqueness, and worth of each develop new buildings and programs. person who walks through our doors. On page 16, you’ll find an update on the five- Thank you for all that you do to support year plan that was ratified by our Board in 2017. Plymouth’s mission, staff, and residents. -

Obituary "P" Index
Obituary "P" Index Copyright © 2004 – 2021 GRHS DISCLAIMER: GRHS cannot guarantee that should you purchase a copy of what you would expect to be an obituary from its obituary collection that you will receive an obituary per se. The obituary collection consists of such items as a) personal cards of information shared with GRHS by researchers, b) www.findagrave.com extractions, c) funeral home cards, d) newspaper death notices, and e) obituaries extracted from newspapers and other publications as well as funeral home web sites. Some obituaries are translations of obituaries published in German publications, although generally GRHS has copies of the German versions. These German versions would have to be ordered separately for they are kept in a separate file in the GRHS library. The list of names and dates contained herein is an alphabetical listing [by surname and given name] of the obituaries held at the Society's headquarters for the letter combination indicated. Each name is followed by the birth date in the first column and death date in the second. Dates may be extrapolated or provided from another source. Important note about UMLAUTS: Surnames in this index have been entered by our volunteers exactly as they appear in each obituary but the use of characters with umlauts in obits has been found to be inconsistant. For example the surname Büchele may be entered as Buchele or Bahmüller as Bahmueller. This is important because surnames with umlauted characters are placed in alphabetic order after regular characters so if you are just scrolling down this sorted list you may find the surname you are looking for in an unexpected place (i.e. -

Actin-Bundling Proteins in Cancer Progression at a Glance
Cell Science at a Glance 1073 Actin-bundling proteins cellular structures, such as filopodia general, crosslinking proteins have two (spike-like protrusions), lamellipodia actin-binding sites, often because they in cancer progression at (sheet-like protrusions), stress fibers (elastic dimerise, and the location of actin-binding a glance contractile bundles), microvilli (finger-like sites determines the filament arrangement surface protrusions) and invadopodia and type of crosslinked structure formed. (invasive cell feet) (see Table 1 for a more Actin filaments are polar, with a fast- Richard P. Stevenson, Douwe complete list). Whereas the cytoskeleton is growing and a slow-growing end, and this Veltman and Laura M. Machesky* important in normal cellular function, it can polarity is maintained by a cycle of ATP The Beatson Institute for Cancer Research, Garscube be subverted in cancer cells and contributes hydrolysis (see Poster) (Pollard and Cooper, Estate, Switchback Rd, Bearsden, Glasgow G61 to changes in cell growth, stiffness, 2009). Bundling proteins can be selective 1BD, UK *Author for correspondence movement and invasiveness. We hereby about the orientation with which they bind to ([email protected]) give an overview of the role of actin- the filament, allowing the specific formation filament bundling in cellular structures and of bundles of either mixed or uniform Journal of Cell Science 125, 1073–1079 ß 2012. Published by The Company of Biologists Ltd discuss how alterations in the activity or polarity (see Poster). Bundling proteins are doi: 10.1242/jcs.093799 expression patterns of actin-bundling often modular and contain repeated actin- proteins could be linked to cancer initiation filament-binding domains (see Poster). -

Solution Structure of the Calponin CH Domain and Fitting to the 3D-Helical Reconstruction of F-Actin:Calponin
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Structure, Vol. 10, 249–258, February, 2002, 2002 Elsevier Science Ltd. All rights reserved. PII S0969-2126(02)00703-7 Solution Structure of the Calponin CH Domain and Fitting to the 3D-Helical Reconstruction of F-Actin:Calponin Janice Bramham,1,6 Julie L. Hodgkinson,2 tropomyosin, caldesmon, and calponin. Basic calponin, Brian O. Smith,3 Dusan Uhrı´n,4 Paul N. Barlow,3,4 one of three genetic variants found almost exclusively in and Steven J. Winder5 smooth muscle, was first isolated from chicken gizzard 1 Department of Biochemistry smooth muscle as a 34 kDa protein that binds to filamen- University of Leicester tous actin (F-actin) [1]. It has been proposed that cal- Adrian Building ponin has a role both in muscle contraction and as a University Road structural component in the cytoskeleton of smooth Leicester LE1 7RH muscle cells. Calponin is associated with the contractile United Kingdom apparatus, along with actin, myosin, and caldesmon. It 2 Imperial College School of Science also occurs in the membrane skeleton that forms the Technology and Medicine interface between the contractile apparatus and the ex- National Heart and Lung Institute tracellular matrix, and it appears in the cytoskeleton, Dovehouse Street which surrounds and supports the contractile appara- London SW3 6LY tus, along with -actin, filamin, and desmin [2]. United Kingdom In vitro studies have shown that calponin is an actin 3 Institute of Cell and Molecular Biology binding protein and a major regulator of muscle contrac- University of Edinburgh tion. -

Transforming Lives, Communities and the World
FOR THE YEAR ENDED THE JUNE 30, 2018 FLAGSHIP DIFFERENCE TRANSFORMING LIVES, COMMUNITIES AND THE WORLD THE UNIVERSITY OF MISSISSIPPI FOUNDATION ANNUAL REPORT ON PHILANTHROPY TOTAL 38% ENDOWMENT BENEFITING THE UNIVERSITY OF MISSISSIPPI $713 19% MILLION ACADEMIC AND PROGRAM SUPPORT 4% FACULTY SUPPORT LIBRARY SUPPORT 39% SCHOLARSHIP SUPPORT The figures represent new gifts and new pledges in the fiscal RECENT $ years in which they were made. PRIVATE SUPPORT 167.6 IN MILLIONS $132.1 $116.4 $116.6 $105.1 $100.2 2013 2014 2015 2016 2017 2018 PUBLISHERS: Wendell W. Weakley Sandra McGuire Guest OF University of Mississippi Foundation EDITOR: TABLE CONTENTS Tina H. Hahn THE ANNUAL REPORT ON PHILANTHROPY FOR THE YEAR ENDED ASSOCIATE EDITORS: JUNE 30, 2018 Bill Dabney Donna Patton MESSAGE FROM MESSAGE FROM EDITORIAL CONSULTANTS: THE CHANCELLOR .........................................................3 THE VICE CHANCELLOR FOR HEALTH AFFAIRS .............43 Hailey Gilleland Wesley Owen MESSAGE FROM UMMC: ELEVATING HEALTH SERVICES, WRITERS: THE UM FOUNDATION PRESIDENT ..................................5 Da’Ron Brown CAMPAIGN DIRECTED TO PEDIATRICS .................45 Bill Dabney INTRODUCTION: UMMC Academic Leadership ......................................51 Tom Fortner TRANSFORMING INDIVIDUALS, Tina Hahn COMMUNITIES AND THE WORLD .............................7 UMMC Development Staff ...........................................53 Lisa Stone CONTRIBUTORS: Major Gifts ..................................................................13 Kyle Campbell MESSAGE