Crassula Genus Plants Response to Temperature Stress Depends on Anatomical Structure and Antioxidant System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plethora of Plants - Collections of the Botanical Garden, Faculty of Science, University of Zagreb (2): Glasshouse Succulents
NAT. CROAT. VOL. 27 No 2 407-420* ZAGREB December 31, 2018 professional paper/stručni članak – museum collections/muzejske zbirke DOI 10.20302/NC.2018.27.28 PLETHORA OF PLANTS - COLLECTIONS OF THE BOTANICAL GARDEN, FACULTY OF SCIENCE, UNIVERSITY OF ZAGREB (2): GLASSHOUSE SUCCULENTS Dubravka Sandev, Darko Mihelj & Sanja Kovačić Botanical Garden, Department of Biology, Faculty of Science, University of Zagreb, Marulićev trg 9a, HR-10000 Zagreb, Croatia (e-mail: [email protected]) Sandev, D., Mihelj, D. & Kovačić, S.: Plethora of plants – collections of the Botanical Garden, Faculty of Science, University of Zagreb (2): Glasshouse succulents. Nat. Croat. Vol. 27, No. 2, 407- 420*, 2018, Zagreb. In this paper, the plant lists of glasshouse succulents grown in the Botanical Garden from 1895 to 2017 are studied. Synonymy, nomenclature and origin of plant material were sorted. The lists of species grown in the last 122 years are constructed in such a way as to show that throughout that period at least 1423 taxa of succulent plants from 254 genera and 17 families inhabited the Garden’s cold glass- house collection. Key words: Zagreb Botanical Garden, Faculty of Science, historic plant collections, succulent col- lection Sandev, D., Mihelj, D. & Kovačić, S.: Obilje bilja – zbirke Botaničkoga vrta Prirodoslovno- matematičkog fakulteta Sveučilišta u Zagrebu (2): Stakleničke mesnatice. Nat. Croat. Vol. 27, No. 2, 407-420*, 2018, Zagreb. U ovom članku sastavljeni su popisi stakleničkih mesnatica uzgajanih u Botaničkom vrtu zagrebačkog Prirodoslovno-matematičkog fakulteta između 1895. i 2017. Uređena je sinonimka i no- menklatura te istraženo podrijetlo biljnog materijala. Rezultati pokazuju kako je tijekom 122 godine kroz zbirku mesnatica hladnog staklenika prošlo najmanje 1423 svojti iz 254 rodova i 17 porodica. -
4.5" Tall = 10 Per; Square One= 8 Per Availability
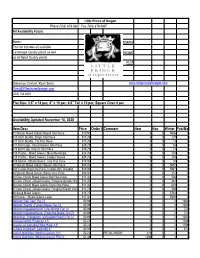
Little Prince of Oregon Phone (503) 678-5687 Fax (503) 678-5887 All Availability Future Notes: Company This list includes all available Landscape Quality plants as well Manager as all Retail Quality plants. PO # Salesman Contact: Ryan Seely www.littleprinceoforegon.com [email protected] (503) 734-6361 Flat Size: 3.5" = 18 per; 4" = 15 per; 4.5" Tall = 10 per; Square One= 8 per Availability Updated November 18, 2020 Item Desc Price Order QtyComment Now Nov Winter Feb/March #1 Wreath Mixed Greens Round One Piece $19.50 0 0 5606 0 #10 Birch Bundle, Single One Piece $13.25 0 0 237 0 #11 Birch Bundle, Trio One Piece $13.25 0 0 13 0 #12 Bird Cage - Mixed Greens One Piece $29.25 0 0 53 0 #13 Bird Cage, Natural One Piece $18.25 0 0 13 0 #14 Planter - Mixed Greens, Metal Rectangle $24.00 0 0 176 0 #15 Planter - Mixed Greens, Copper Square $28.75 0 0 283 0 #16 Basket - Mixed Greens, Tree One Piece $10.00 0 0 35 0 #2 Wreath Mixed Greens Square One Piece $20.25 0 0 5652 0 #20 Candle Ring One Piece, Candle NOT Included $10.00 0 0 5881 0 #3 Wreath Mixed Greens Wicker One Piece $26.50 0 0 215 0 #4 Door Charm Mixed Greens Bell One Piece $11.00 0 0 506 0 #5 Door Charm - Mixed Greens, Pinecone Bundle One Piece$12.25 0 0 242 0 #6 Door Charm Mixed Greens Cone One Piece $11.00 0 0 629 0 #7 Door Charm - Mixed Greens, Hanging Wreath One Piece$13.75 0 0 298 0 #8 Swag Mixed Greens $15.75 0 0 5784 0 #9 Runner - Mixed Greens Large $27.00 0 0 5908 0 Abutilon 'Red Tiger' Flat 72 $0.89 0 0 0 1080 Abutilon 'Savitzii' (Parlour Maple) Flat 72 $0.89 0 0 0 1080 Abutilon magapotamicum 'Little Shrimp' Flat 72 $0.89 0 0 0 720 Abutilon megapotamicum (Flowering Maple) Flat 72 $0.89 0 0 0 720 Abutilon p. -

Kruiedokters, Plants and Molecules: Relations of Power, Wind, and Matter in Namaqualand
Kruiedokters, plants and molecules: relations of power, wind, and matter in Namaqualand Joshua B. Cohen (CHNJOS009) Thesis Presented for the Degree of DOCTOR OF PHILOSOPHY Anthropology Section, School of African and Gender Studies, Anthropology and Linguistics UNIVERSITY OF CAPE TOWN February 2015 University of Cape Town 1 The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University of Cape Town Acknowledgements A heartfelt and appreciative thank you to every single person in the Kamiesberg (and those encountered on fieldwork trips further afield) who gave me the time of day, who shared cups of tea, wine, beer, stories, anecdotes, laughs and arguments. All those who afforded me time out of their lives to speak with me, to help me understand in some way the realities described in this thesis. I recognise that many people in the Kamiesberg actively deny the reality of many of the ‘magical’ forces and practices detailed in this thesis. On the otherhand, these are very real presences in the lives of other people in the area whom I have come to know. Because of this, I want to stress that I am only expressing my understanding of these realities from the perspective of some people in the Kamiesberg. In particular, I want to thank “Koos”, a kruiedokter (literally ‘herb doctor’) who welcomed me into his home, who treated me like a huiskind (home child) and spent so many, many hours teaching me about herbs, about his work, and who allowed me to sit in on so many of his consultations. -

Koenabib Mine Near Aggeneys, Northern Cape Province
KOENABIB MINE NEAR AGGENEYS, NORTHERN CAPE PROVINCE BOTANICAL STUDY AND ASSESSMENT Version: 1.0 Date: 30th January 2020 Authors: Gerhard Botha & Dr. Jan -Hendrik Keet PROPOSED MINING OF SILLIMANITE, AGGREGATE AND GRAVEL ON THE FARM KOENABIB 43 NORTH OF AGGENEYS, NORTHERN CAPE PROVINCE Report Title: Botanical Study and Assessment Authors: Mr. Gerhard Botha & Dr. Jan-Hendrik Keet Project Name: Proposed Mining of Sillimanite, Aggregate and Gravel on the Farm Koenabib 43, North of Aggeneys, Northern Cape Province Status of report: Version 1.0 Date: 30th January 2020 Prepared for: Greenmined Environmental Postnet Suite 62, Private Bag X15 Somerset West 7129 Cell: 082 734 5113 Email: [email protected] Prepared by Nkurenkuru Ecology and Biodiversity 3 Jock Meiring Street Park West Bloemfontein 9301 Cell: 083 412 1705 Email: gabotha11@gmail com Suggested report citation Nkurenkuru Ecology and Biodiversity, 2019. Mining Permit, Final Basic Assessment & Environmental Management Plan for the proposed mining of Sillimanite, Aggregate and Stone Gravel on the Farm Koenabib 43, Northern Cape Province. Botanical Study and Assessment Report. Unpublished report prepared by Nkurenkuru Ecology and Biodiversity for GreenMined Environmental. Version 1.0, 30 January 2020. Proposed koenabib sillimanite mine, NORTHERN CAPE PROVINCE January 2020 botanical STUDY AND ASSESSMENT I. DECLARATION OF CONSULTANTS INDEPENDENCE » act/ed as the independent specialist in this application; » regard the information contained in this report as it relates to my specialist -

Vascular Plants of Santa Cruz County, California
ANNOTATED CHECKLIST of the VASCULAR PLANTS of SANTA CRUZ COUNTY, CALIFORNIA SECOND EDITION Dylan Neubauer Artwork by Tim Hyland & Maps by Ben Pease CALIFORNIA NATIVE PLANT SOCIETY, SANTA CRUZ COUNTY CHAPTER Copyright © 2013 by Dylan Neubauer All rights reserved. No part of this publication may be reproduced without written permission from the author. Design & Production by Dylan Neubauer Artwork by Tim Hyland Maps by Ben Pease, Pease Press Cartography (peasepress.com) Cover photos (Eschscholzia californica & Big Willow Gulch, Swanton) by Dylan Neubauer California Native Plant Society Santa Cruz County Chapter P.O. Box 1622 Santa Cruz, CA 95061 To order, please go to www.cruzcps.org For other correspondence, write to Dylan Neubauer [email protected] ISBN: 978-0-615-85493-9 Printed on recycled paper by Community Printers, Santa Cruz, CA For Tim Forsell, who appreciates the tiny ones ... Nobody sees a flower, really— it is so small— we haven’t time, and to see takes time, like to have a friend takes time. —GEORGIA O’KEEFFE CONTENTS ~ u Acknowledgments / 1 u Santa Cruz County Map / 2–3 u Introduction / 4 u Checklist Conventions / 8 u Floristic Regions Map / 12 u Checklist Format, Checklist Symbols, & Region Codes / 13 u Checklist Lycophytes / 14 Ferns / 14 Gymnosperms / 15 Nymphaeales / 16 Magnoliids / 16 Ceratophyllales / 16 Eudicots / 16 Monocots / 61 u Appendices 1. Listed Taxa / 76 2. Endemic Taxa / 78 3. Taxa Extirpated in County / 79 4. Taxa Not Currently Recognized / 80 5. Undescribed Taxa / 82 6. Most Invasive Non-native Taxa / 83 7. Rejected Taxa / 84 8. Notes / 86 u References / 152 u Index to Families & Genera / 154 u Floristic Regions Map with USGS Quad Overlay / 166 “True science teaches, above all, to doubt and be ignorant.” —MIGUEL DE UNAMUNO 1 ~ACKNOWLEDGMENTS ~ ANY THANKS TO THE GENEROUS DONORS without whom this publication would not M have been possible—and to the numerous individuals, organizations, insti- tutions, and agencies that so willingly gave of their time and expertise. -

Flora-Lab-Manual.Pdf
LabLab MManualanual ttoo tthehe Jane Mygatt Juliana Medeiros Flora of New Mexico Lab Manual to the Flora of New Mexico Jane Mygatt Juliana Medeiros University of New Mexico Herbarium Museum of Southwestern Biology MSC03 2020 1 University of New Mexico Albuquerque, NM, USA 87131-0001 October 2009 Contents page Introduction VI Acknowledgments VI Seed Plant Phylogeny 1 Timeline for the Evolution of Seed Plants 2 Non-fl owering Seed Plants 3 Order Gnetales Ephedraceae 4 Order (ungrouped) The Conifers Cupressaceae 5 Pinaceae 8 Field Trips 13 Sandia Crest 14 Las Huertas Canyon 20 Sevilleta 24 West Mesa 30 Rio Grande Bosque 34 Flowering Seed Plants- The Monocots 40 Order Alistmatales Lemnaceae 41 Order Asparagales Iridaceae 42 Orchidaceae 43 Order Commelinales Commelinaceae 45 Order Liliales Liliaceae 46 Order Poales Cyperaceae 47 Juncaceae 49 Poaceae 50 Typhaceae 53 Flowering Seed Plants- The Eudicots 54 Order (ungrouped) Nymphaeaceae 55 Order Proteales Platanaceae 56 Order Ranunculales Berberidaceae 57 Papaveraceae 58 Ranunculaceae 59 III page Core Eudicots 61 Saxifragales Crassulaceae 62 Saxifragaceae 63 Rosids Order Zygophyllales Zygophyllaceae 64 Rosid I Order Cucurbitales Cucurbitaceae 65 Order Fabales Fabaceae 66 Order Fagales Betulaceae 69 Fagaceae 70 Juglandaceae 71 Order Malpighiales Euphorbiaceae 72 Linaceae 73 Salicaceae 74 Violaceae 75 Order Rosales Elaeagnaceae 76 Rosaceae 77 Ulmaceae 81 Rosid II Order Brassicales Brassicaceae 82 Capparaceae 84 Order Geraniales Geraniaceae 85 Order Malvales Malvaceae 86 Order Myrtales Onagraceae -

CHAPTER 12 SPECIES TREATMENT (Enumeration of the 220 Obligate Or Near-Obligate Cremnophilous Succulent and Bulbous Taxa) FERNS P
CHAPTER 12 SPECIES TREATMENT (Enumeration of the 220 obligate or near-obligate cremnophilous succulent and bulbous taxa) FERNS POLYPODIACEAE Pyrrosia Mirb. 1. Pyrrosia schimperiana (Mett. ex Kuhn) Alston PYRROSIA Mirb. 1. Pyrrosia schimperiana (Mett. ex Kuhn) Alston in Journal of Botany, London 72, Suppl. 2: 8 (1934). Cremnophyte growth form: Cluster-forming, subpendulous leaves (of medium weight, cliff hugger). Growth form formula: A:S:Lper:Lc:Ts (p) Etymology: After Wilhelm Schimper (1804–1878), plant collector in northern Africa and Arabia. DESCRIPTION AND HABITAT Cluster-forming semipoikilohydric plant, with creeping rhizome 2 mm in diameter; rhizome scales up to 6 mm long, dense, ovate-cucullate to lanceolate-acuminate, entire. Fronds ascending-spreading, becoming pendent, 150–300 × 17–35 mm, succulent-coriaceous, closely spaced to ascending, often becoming drooping (2–6 mm apart); stipe tomentose (silvery grey to golden hairs), becoming glabrous with age. Lamina linear-lanceolate to linear-obovate, rarely with 1 or 2 lobes; margin entire; adaxial surface tomentose becoming glabrous, abaxial surface remaining densely tomentose (grey to golden stellate hairs); base cuneate; apex acute. Sori rusty brown dots, 1 mm in diameter, evenly spaced (1–2 mm apart) in distal two thirds on abaxial surface, emerging through dense indumentum. Phenology: Sori produced mainly in summer and spring. Spores dispersed by wind, coinciding with the rainy season. Habitat and aspect: Sheer south-facing cliffs and rocky embankments, among lichens and other succulent flora. Plants are scattered, firmly rooted in crevices and on ledges. The average daily maximum temperature is about 26ºC for summer and 14ºC for winter. Rainfall is experienced mainly in summer, 1000–1250 mm per annum. -

Prickly News South Coast Cactus & Succulent Society Newsletter | March 2021
PRICKLY NEWS SOUTH COAST CACTUS & SUCCULENT SOCIETY NEWSLETTER | MARCH 2021 Gary ZOOM PRESENTATION SHARE YOUR GARDEN OR YOUR FAVORITE PLANT Duke Sunday, March 14 @ 1:30 pm “Chile – Land of the Ancients” I hope you are all staying well. The unseasonably warm weather has given me lots of work to do with repotting and watering. I was counting on the winter rains to do some of my work, but alas, not so. Email me with photos of your garden and/or plants Usually at this time of year we are preparing for that we can publish as a way of staying connected. the Annual Show and Sale which will not take place this year. [email protected] This may give us more time to work on our plants. And, if you have extra plants, you could save them for a future sale. CALL FOR PHOTOS: The Mini-show genera for March are To learn more visit southcoastcss.org Cactus: Echinocereus and Succulent: Gasteria and hybrids (so that includes Gasteraloes, Gastroworthias, etc.) Photos will be published Like us on our facebook page and you will be given one Mini-show point each for a submitted photo of your cactus, succulent or garden (up to 2 points). Please include your plant’s full name if you know it (and if you don’t, Follow us on Instagram, _sccss_ I will seek advice for you). Let me know if you would prefer not to have your name published with the photos. The photos should be as high resolution as possible so they will publish well and should show IN THIS ISSUE off the plant as you would in a Mini-show. -

Checklist of the Vascular Plants of San Diego County 5Th Edition
cHeckliSt of tHe vaScUlaR PlaNtS of SaN DieGo coUNty 5th edition Pinus torreyana subsp. torreyana Downingia concolor var. brevior Thermopsis californica var. semota Pogogyne abramsii Hulsea californica Cylindropuntia fosbergii Dudleya brevifolia Chorizanthe orcuttiana Astragalus deanei by Jon P. Rebman and Michael G. Simpson San Diego Natural History Museum and San Diego State University examples of checklist taxa: SPecieS SPecieS iNfRaSPecieS iNfRaSPecieS NaMe aUtHoR RaNk & NaMe aUtHoR Eriodictyon trichocalyx A. Heller var. lanatum (Brand) Jepson {SD 135251} [E. t. subsp. l. (Brand) Munz] Hairy yerba Santa SyNoNyM SyMBol foR NoN-NATIVE, NATURaliZeD PlaNt *Erodium cicutarium (L.) Aiton {SD 122398} red-Stem Filaree/StorkSbill HeRBaRiUM SPeciMeN coMMoN DocUMeNTATION NaMe SyMBol foR PlaNt Not liSteD iN THE JEPSON MANUAL †Rhus aromatica Aiton var. simplicifolia (Greene) Conquist {SD 118139} Single-leaF SkunkbruSH SyMBol foR StRict eNDeMic TO SaN DieGo coUNty §§Dudleya brevifolia (Moran) Moran {SD 130030} SHort-leaF dudleya [D. blochmaniae (Eastw.) Moran subsp. brevifolia Moran] 1B.1 S1.1 G2t1 ce SyMBol foR NeaR eNDeMic TO SaN DieGo coUNty §Nolina interrata Gentry {SD 79876} deHeSa nolina 1B.1 S2 G2 ce eNviRoNMeNTAL liStiNG SyMBol foR MiSiDeNtifieD PlaNt, Not occURRiNG iN coUNty (Note: this symbol used in appendix 1 only.) ?Cirsium brevistylum Cronq. indian tHiStle i checklist of the vascular plants of san Diego county 5th edition by Jon p. rebman and Michael g. simpson san Diego natural history Museum and san Diego state university publication of: san Diego natural history Museum san Diego, california ii Copyright © 2014 by Jon P. Rebman and Michael G. Simpson Fifth edition 2014. isBn 0-918969-08-5 Copyright © 2006 by Jon P. -

Catalogue 10 05 2012.Xlsx
CATALOGUE LISTE DES REFERENCES Pépinière Arrée Succulentes CATALOGUE ‐ LISTE DES REFERENCES T N E N N IO E O G I A T IT A T P E N I L E N A IC E R L N R S I I T I O T C E F M M G S IL P N S V E O A RI U I T X O R N DE F O R H U E C 0007 Adromischus cooperii Plante compacte aux feuilles très charnue. Forme un petit coussin Crassulaceae Afrique du -2°C 5-10°C Composition - Soleil Pot de 8 cm de 15cm. Sud Isolé 0146 Adromischus maculatus Jolie petite plante compacte aux feuilles vertes tachetées de brun, et Crassulaceae Afrique du -2°C 5-10°C Mi-ombre / Soleil Pot de 8 cm disposées de manière à lui donner une forme de cristaux. Done ses Sud Ombre meuilleures couleurs à l'ombre. 0122 Aeonium arboreum Port arbustif, atteignant environ 1m de hauteur. Tiges ramifiées, Crassulaceae Iles Canaries -4°C 0-5°C isolé Soleil - mi Pot de 9 cm feuillage persistant composé de feuilles disposées en rosettes au ombre sommet de ces tiges. Floraison jaune, au printemps. 0111 Aeonium arboreum 'Holochrysum' Aeonium au port arbustif pouvant atteindre 1 ou 2 m de hauteur. Crassulaceae Iles Canaries -4°C 0-5°C isolé Soleil - mi Pot de 8 cm Feuillage vert jaune et brillant. ombre 0003 Aeonium arboreum 'Schwartzkopf' Un cultivar issu de Aeonium arboreum var. atropurpureum. Feuillage Crassulaceae Horticole -4°C 5-10°C isolé Soleil Pot de 9 cm très foncé presque noir et brillant. -

Facultative Crassulacean Acid Metabolism (CAM) Plants: Powerful Tools for Unravelling the Functional Elements of CAM Photosynthesis
Journal of Experimental Botany Advance Access published March 18, 2014 Journal of Experimental Botany doi:10.1093/jxb/eru063 REVIEW PAPER Facultative crassulacean acid metabolism (CAM) plants: powerful tools for unravelling the functional elements of CAM photosynthesis Klaus Winter1,* and Joseph A. M. Holtum1,2 1 Smithsonian Tropical Research Institute, PO Box 0843-03092, Balboa, Ancon, Republic of Panama 2 School of Marine and Tropical Biology, James Cook University, Townsville, Queensland 4811, Australia * To whom correspondence should be addressed. E-mail: [email protected] Received 23 September 2013; Revised 24 January 2014; Accepted 29 January 2014 Downloaded from Abstract Facultative crassulacean acid metabolism (CAM) describes the optional use of CAM photosynthesis, typically under http://jxb.oxfordjournals.org/ conditions of drought stress, in plants that otherwise employ C3 or C4 photosynthesis. In its cleanest form, the upregu- lation of CAM is fully reversible upon removal of stress. Reversibility distinguishes facultative CAM from ontogeneti- cally programmed unidirectional C3-to-CAM shifts inherent in constitutive CAM plants. Using mainly measurements of 24 h CO2 exchange, defining features of facultative CAM are highlighted in five terrestrial species, Clusia pratensis, Calandrinia polyandra, Mesembryanthemum crystallinum, Portulaca oleracea and Talinum triangulare. For these, we provide detailed chronologies of the shifts between photosynthetic modes and comment on their usefulness as experimental systems. Photosynthetic flexibility is also reviewed in an aquatic CAM plant, Isoetes howellii. Through by guest on March 19, 2014 comparisons of C3 and CAM states in facultative CAM species, many fundamental biochemical principles of the CAM pathway have been uncovered. Facultative CAM species will be of even greater relevance now that new sequencing technologies facilitate the mapping of genomes and tracking of the expression patterns of multiple genes. -

Succulent Standards
SUCCULENT STANDARDS JDGZ, EPS-URL & EDY TOBAR V1.0 2018 VARIEDADES ADROMISCHUS CRISTATUS URC CRASSULA LACTEA URC ADROMISCHUS FILICAULIS URC CRASSULA MARNIERIANA MINOR ADROMISCHUS MACULATUS URC CRASSULA MARNIERIANA URC AEONIUM KIWI URC CRASSULA MESEMBRYANTHEMOIDES AEONIUM KIWI VERDE URC CRASSULA MONEY MAKER® URC AEONIUM ZWARTKOP URC CRASSULA MUSCOSA URC ALOE BLUE ELF URC CRASSULA OBVALLATA URC ALOE DOROTHEA URC CRASSULA OVATA URC ALOE PINK BLUSH URC CRASSULA OVATA CORAL URC CARALLUMA CAUDATA URC CRASSULA OVATA MINI URC CRASSULA PAGODA URC (4x300) CRASSULA ARBORESCENS BLUE HALE URC CRASSULA PERFORATA STRING OF CRASSULA ARBORESCENS CURLY GREEN BUTTONS URC CRASSULA ARBORESCENS URC CRASSULA PORTULACEA IBRIDA CRASSULA BREVIFOLIA URC CRASSULA RED CORAL URC CRASSULA CALICO KITTEN CRASSULA RUPESTRIS URC CRASSULA CAMPFIRE URC CRASSULA SARMENTOSA URC CRASSULA CEPHALOPHORA URC CRASSULA SOCIALIS URC CRASSULA SPRINGTIME URC CRASSULA COMMUTATA URC CYANOTIS SOMALENSIS URC CRASSULA CORYMBULOSA SHARK'S TOOTH CRASSULA FALCATA URC DELOSPERMA ORANGE HARDY ICE PLANT CRASSULA HOBBIT URC DELOSPERMA SP. LEHMANNII URC VARIEDADES ECHEVERIA HYBRID DUSTY PINK URC ECHEVERIA GRAPTOPETALUM FILIFERUM URC ECHEVERIA HYBRID EBONY ECHEVERIA GRAPTOPETALUM MACDOUGALLII ECHEVERIA HYBRID ELEGANS URC ECHEVERIA GRAPTOPETALUM PARAGUAYENSE MINOR ECHEVERIA HYBRID FABIOLA ECHEVERIA GRAPTOVERIA FRED IVES ECHEVERIA HYBRID GIBBIFLORA URC ECHEVERIA HYBRID "TOPSY TURVY" URC ECHEVERIA HYBRID GLOBULOSA URC ECHEVERIA HYBRID AFFINIS URC ECHEVERIA HYBRID AFTERGLOW URC ECHEVERIA HYBRID GRAPTOVERIA