Cervical Cytology and Vulvar Pathology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FGM in Canada
Compiled by Patricia Huston MD, MPH Scientific Communications International, Inc for the Federal Interdepartmental Working Group on FGM. Copies of this report are available from: Women's Health Bureau Health Canada [email protected] The Canadian Women's Health Network 203-419 Graham Avenue Winnipeg, Manitoba R3C 0M3 fax: (204)989-2355 The opinions expressed in this report are not necessarily those of the Government of Canada or any of the other organizations represented. Dedication This report is dedicated to all the women in the world who have undergone FGM and to all the people who are helping them live with and reverse this procedure. This report is part of the ongoing commitment of Canadians and the Government of Canada to stop this practice in Canada and to improve the health and well-being of affected women and their communities. Executive Summary Female genital mutilation (FGM), or the ritual excision of part or all of the external female genitalia, is an ancient cultural practice that occurs around the world today, especially in Africa. With recent immigration to Canada of peoples from Somalia, Ethiopia and Eritrea, Sudan and Nigeria, women who have undergone this practice are now increasingly living in Canada. It is firmly believed by the people who practise it, that FGM improves feminine hygiene, that it will help eliminate disease and it is thought to be the only way to preserve family honour, a girl's virginity and her marriageability. FGM has a number of important adverse health effects including risks of infection and excessive bleeding (often performed when a girl is pre-pubertal). -

Core Curriculum for Surgical Technology Sixth Edition
Core Curriculum for Surgical Technology Sixth Edition Core Curriculum 6.indd 1 11/17/10 11:51 PM TABLE OF CONTENTS I. Healthcare sciences A. Anatomy and physiology 7 B. Pharmacology and anesthesia 37 C. Medical terminology 49 D. Microbiology 63 E. Pathophysiology 71 II. Technological sciences A. Electricity 85 B. Information technology 86 C. Robotics 88 III. Patient care concepts A. Biopsychosocial needs of the patient 91 B. Death and dying 92 IV. Surgical technology A. Preoperative 1. Non-sterile a. Attire 97 b. Preoperative physical preparation of the patient 98 c. tneitaP noitacifitnedi 99 d. Transportation 100 e. Review of the chart 101 f. Surgical consent 102 g. refsnarT 104 h. Positioning 105 i. Urinary catheterization 106 j. Skin preparation 108 k. Equipment 110 l. Instrumentation 112 2. Sterile a. Asepsis and sterile technique 113 b. Hand hygiene and surgical scrub 115 c. Gowning and gloving 116 d. Surgical counts 117 e. Draping 118 B. Intraoperative: Sterile 1. Specimen care 119 2. Abdominal incisions 121 3. Hemostasis 122 4. Exposure 123 5. Catheters and drains 124 6. Wound closure 128 7. Surgical dressings 137 8. Wound healing 140 1 c. Light regulation d. Photoreceptors e. Macula lutea f. Fovea centralis g. Optic disc h. Brain pathways C. Ear 1. Anatomy a. External ear (1) Auricle (pinna) (2) Tragus b. Middle ear (1) Ossicles (a) Malleus (b) Incus (c) Stapes (2) Oval window (3) Round window (4) Mastoid sinus (5) Eustachian tube c. Internal ear (1) Labyrinth (2) Cochlea 2. Physiology of hearing a. Sound wave reception b. Bone conduction c. -
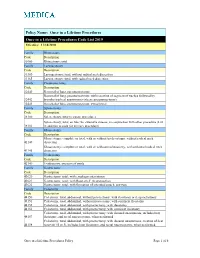
Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010
Policy Name: Once in a Lifetime Procedures Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010 Family Rhinectomy Code Description 30160 Rhinectomy; total Family Laryngectomy Code Description 31360 Laryngectomy; total, without radical neck dissection 31365 Laryngectomy; total, with radical neck dissection Family Pneumonectomy Code Description 32440 Removal of lung, pneumonectomy; Removal of lung, pneumonectomy; with resection of segment of trachea followed by 32442 broncho-tracheal anastomosis (sleeve pneumonectomy) 32445 Removal of lung, pneumonectomy; extrapleural Family Splenectomy Code Description 38100 Splenectomy; total (separate procedure) Splenectomy; total, en bloc for extensive disease, in conjunction with other procedure (List 38102 in addition to code for primary procedure) Family Glossectomy Code Description Glossectomy; complete or total, with or without tracheostomy, without radical neck 41140 dissection Glossectomy; complete or total, with or without tracheostomy, with unilateral radical neck 41145 dissection Family Uvulectomy Code Description 42140 Uvulectomy, excision of uvula Family Gastrectomy Code Description 43620 Gastrectomy, total; with esophagoenterostomy 43621 Gastrectomy, total; with Roux-en-Y reconstruction 43622 Gastrectomy, total; with formation of intestinal pouch, any type Family Colectomy Code Description 44150 Colectomy, total, abdominal, without proctectomy; with ileostomy or ileoproctostomy 44151 Colectomy, total, abdominal, without proctectomy; with continent ileostomy 44155 Colectomy, -

GENDER DYSPHORIA TREATMENT Policy Number: 2016T0580A Effective Date: January 1, 2017
UnitedHealthcare® Commercial Medical Policy GENDER DYSPHORIA TREATMENT Policy Number: 2016T0580A Effective Date: January 1, 2017 Table of Contents Page Related Commercial Policy INSTRUCTIONS FOR USE .......................................... 1 Blepharoplasty, Blepharoptosis and Brow Ptosis BENEFIT CONSIDERATIONS ...................................... 1 Repair COVERAGE RATIONALE ............................................. 2 Botulinum Toxin A and B DEFINITIONS .......................................................... 3 Cosmetic and Reconstructive Procedures APPLICABLE CODES ................................................. 4 DESCRIPTION OF SERVICES ...................................... 8 Gonadotropin Releasing Hormone Analogs CLINICAL EVIDENCE ................................................. 8 Panniculectomy and Body Contouring Procedures U.S. FOOD AND DRUG ADMINISTRATION ................... 11 Rhinoplasty and Other Nasal Surgeries CENTERS FOR MEDICARE AND MEDICAID SERVICES ... 11 Speech Language Pathology Services REFERENCES .......................................................... 11 POLICY HISTORY/REVISION INFORMATION ................ 12 Community Plan Policy Gender Dysphoria Treatment Related Optum Guideline Gender Dysphoria INSTRUCTIONS FOR USE This Medical Policy provides assistance in interpreting UnitedHealthcare benefit plans. When deciding coverage, the member specific benefit plan document must be referenced. The terms of the member specific benefit plan document [e.g., Certificate of Coverage (COC), Schedule of -

April 2013, Volume 5, Issue 4
VOLUME 5 MONTHLY ISSUE MEDICAL STAFF 4 NEWSLETTER ProgressNotes April TORRANCE MEMORIAL MEDICAL CENTER 2013 this issue CPOE P.1 Doctor’s Day P.2 MEC Approvals P.3-4 Medical Staff Calendar P.5 New Providers P.6 Roster Updates P.6 What is it? CPOE Select Rewards is a tiered program geared to reward pro- CPOE viders as they expand their use of CPOE (Computerized Provider Order Entry). Computerized How are providers enrolled in the CPOE Select Rewards Provider Order Program? Entry Select Enrollment is automatic as you or your group begins CPOE. Rewards Program How does it work? Providers qualify for monthly raffles based on meeting or exceed- ing CPOE percentage goals for their assigned tier. When will winners be announced? Winners will be announced the second Monday of every month, beginning in April. CPOE Monthly Raffle includes several prizes including an i-Pad mini. Individualized program details will be sent out to providers. Please call CPOE hotline at x2763 (xCPOE) if you have any questions. Doctor’s Day Thank you to all the physicians who attended the Doctor’s Day Celebration at Torrance Memorial on Wednesday, March 27th. The event was a success with many physicians attending to enjoy a great meal, music and prizes. Congratulations to Dr. Lauren Nguyen and Dr. James Flores, the two lucky winners of the iPad mini and the thirty physicians who also won gift cards to Nordstroms, California Pizza Kitchen, HealthLinks, Starbucks and iTunes. Torrance Memorial appreciates our physicians’ dedication and commitment to the health of our community. Medical Executive Committee Approvals The following items were presented and actions were approved at the March 12, 2013 Medical Executive Committee meeting: A. -

Gender Dysphoria Treatment – Commercial Medical Policy
UnitedHealthcare® Commercial Medical Policy Gender Dysphoria Treatment Policy Number: 2021T0580J Effective Date: April 1, 2021 Instructions for Use Table of Contents Page Related Commercial Policies Coverage Rationale ....................................................................... 1 • Blepharoplasty, Blepharoptosis and Brow Ptosis Documentation Requirements ...................................................... 3 Repair Definitions ...................................................................................... 4 • Botulinum Toxins A and B Applicable Codes .......................................................................... 5 • Cosmetic and Reconstructive Procedures Description of Services ................................................................. 9 • Gonadotropin Releasing Hormone Analogs Benefit Considerations .................................................................. 9 Clinical Evidence ......................................................................... 10 • Habilitative Services and Outpatient Rehabilitation U.S. Food and Drug Administration ........................................... 15 Therapy References ................................................................................... 15 • Panniculectomy and Body Contouring Procedures Policy History/Revision Information ........................................... 16 • Rhinoplasty and Other Nasal Surgeries Instructions for Use ..................................................................... 17 Community Plan Policy • Gender Dysphoria -

Sexuality for the Woman with Cancer
Sexuality for the Woman With Cancer Cancer, sex, and sexuality When you first learned you had cancer, you probably thought mostly of survival. But after a while other questions may have started coming up. You might be wondering “How ‘normal’ can my life be, even if the cancer is under control?” Or even “How will cancer affect my sex life?” It’s important to know that you can get help if you are having sexual problems after cancer treatment. There are many good treatments available. Sex and sexuality are important parts of everyday life. The difference between sex and sexuality is that sex is thought of as an activity – something you do with a partner. Sexuality is more about the way you feel about yourself as a woman, and is linked to intimacy or your need for caring, closeness, and touch. Feelings about sexuality affect our zest for living, our self-image, and our relationships with others. Yet patients and doctors often do not talk about the effects of cancer treatment on a woman’s sex life or how she can address problems she’s having. Why? A person may feel uneasy talking about sex with a professional like a doctor or even with a close sex partner. Many people feel awkward and exposed when talking about sex. This information is for all women who have or have had cancer – regardless of their sexual orientation. We cannot answer every question, but we’ll try to give you enough information to help you and your partner have open, honest talks about intimacy and sex. -

Guidelines for the Diagnosis and Management of Vulval Carcinoma
Guidelines for the Diagnosis and Management of Vulval Carcinoma May 2014 Contents Development of the document 4 Summary of consensus statements 5 1. Background 7 Objectives Methodology Definitions of excision 2. Screening, diagnosis and presentation 9 Screening Presentation National Institute for Health and Care Excellence (NICE) guidance Diagnosis Biopsies 3. Pathology 13 Microscopic Spread Staging Prognosis Histology Clinical information Ancillary studies 4. Treatment of primary disease 16 Surgery Reconstructive surgery Surgical management of nonsquamous vulval cancer Morbidity related to surgery 5. Radiotherapy 23 Primary treatment 2 6. Chemotherapy 25 Neoadjuvant chemotherapy for invasive squamous cell carcinoma Adjuvant chemotherapy for vulval cancer Chemotherapy for metastatic and recurrent vulval cancer Concomitant chemotherapy and radiotherapy Future developments 7. Treatment of recurrent disease 28 Recurrence rates and survival Groin recurrence Chemotherapy for relapsed disease 8. Follow-up 30 References 31 Appendix I 35 3 Development of the document This report is an updated version of that published by the Royal College of Obstetricians and Gynaecologists in January 2006. The following individuals have contributed to previous RCOG editions of this guideline: Professor DM Luesley FRCOG Mr DPJ Barton MRCOG Mr M Bishop FRCS Eng (Consultant Urologist), City Hospital, Nottingham Dr P Blake FRCR (Consultant Radiotherapist) Dr CH Buckley FRCPath (Honorary Consultant Histopathologist) Dr JA Davis FRCOG Mr M Ledwick RGN (Cancer Information -

SJH Procedures
SJH Procedures - Gynecology and Gynecology Oncology Services New Name Old Name CPT Code Service ABLATION, LESION, CERVIX AND VULVA, USING CO2 LASER LASER VAPORIZATION CERVIX/VULVA W CO2 LASER 56501 Destruction of lesion(s), vulva; simple (eg, laser surgery, Gynecology electrosurgery, cryosurgery, chemosurgery) 56515 Destruction of lesion(s), vulva; extensive (eg, laser surgery, Gynecology electrosurgery, cryosurgery, chemosurgery) 57513 Cautery of cervix; laser ablation Gynecology BIOPSY OR EXCISION, LESION, FACE AND NECK EXCISION/BIOPSY (MASS/LESION/LIPOMA/CYST) FACE/NECK General, Gynecology, Plastics, ENT, Maxillofacial BIOPSY OR EXCISION, LESION, FACE AND NECK, 2 OR MORE EXCISE/BIOPSY (MASS/LESION/LIPOMA/CYST) MULTIPLE FACE/NECK 11102 Tangential biopsy of skin (eg, shave, scoop, saucerize, curette); General, Gynecology, single lesion Aesthetics, Urology, Maxillofacial, ENT, Thoracic, Vascular, Cardiovascular, Plastics, Orthopedics 11103 Tangential biopsy of skin (eg, shave, scoop, saucerize, curette); General, Gynecology, each separate/additional lesion (list separately in addition to Aesthetics, Urology, code for primary procedure) Maxillofacial, ENT, Thoracic, Vascular, Cardiovascular, Plastics, Orthopedics 11104 Punch biopsy of skin (including simple closure, when General, Gynecology, performed); single lesion Aesthetics, Urology, Maxillofacial, ENT, Thoracic, Vascular, Cardiovascular, Plastics, Orthopedics 11105 Punch biopsy of skin (including simple closure, when General, Gynecology, performed); each separate/additional lesion -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular -

Gender Reassignment Surgery Model Ncd
GENDER REASSIGNMENT SURGERY MODEL NCD I. Indications, Limitations of Coverage and/or Medical Necessity 1 II. Documentation Requirements 4 III. Providers of Gender Reassignment Surgery 5 IV. Common CPT Codes 5 V. ICD-9 and ICD-10 Codes 8 VI. References 9 Written by Transgender Medicine Model NCD Working Group. Contact: Anand Kalra, Transgender Law Center ([email protected]). Gender Reassignment Surgery Model NCD | 1 I. Indications, Limitations of Coverage and/or Medical Necessity The purpose of this National Coverage Determination is to implement the U.S. Department of Health and Human Services Departmental Appeals Board’s 2014 decision overturning NCD 140.3 (Transsexual Surgery). The U.S. Department of Health and Human Services Departmental Appeals Board (“DAB”) considered categories of evidence as outlined in the Medicare Integrity Program Manual § 13.7.1 when it determined that the previously extant prohibition on “transsexual surgery” in NCD 140.3 was unreasonable.1 Implementing a policy to provide access to Gender Reassignment Surgery is centered in improving population health outcomes among transgender Medicare beneficiaries. The Medicare Integrity Program Manual § 13.7.1 provides that NCDs should be based on published authoritative evidence and general acceptance by the medical community. Summarized, this means treatments should follow: Evidence-based best practice derived from definitive studies such as meta-analyses, randomized clinical trials, or clinical evidence Best practice as accepted by the medical community, as supported by sound medical evidence based on: o Scientific data or research studies published in peer-reviewed medical journals; o Consensus of expert medical opinion (i.e., recognized authorities in the field); or o Medical opinion derived from consultations with medical associations or other health care experts. -

Outpatient Surgical Procedures – Site of Service: CPT/HCPCS Codes
UnitedHealthcare® Commercial Policy Appendix: Applicable Code List Outpatient Surgical Procedures – Site of Service: CPT/HCPCS Codes This list of codes applies to the Utilization Review Guideline titled Effective Date: August 1, 2021 Outpatient Surgical Procedures – Site of Service. Applicable Codes The following list(s) of procedure and/or diagnosis codes is provided for reference purposes only and may not be all inclusive. The listing of a code does not imply that the service described by the code is a covered or non-covered health service. Benefit coverage for health services is determined by the member specific benefit plan document and applicable laws that may require coverage for a specific service. The inclusion of a code does not imply any right to reimbursement or guarantee claim payment. Other Policies and Guidelines may apply. This list contains CPT/HCPCS codes for the following: • Auditory System • Female Genital System • Musculoskeletal System • Cardiovascular System • Hemic and Lymphatic Systems • Nervous System • Digestive System • Integumentary System • Respiratory System • Eye/Ocular Adnexa System • Male Genital System • Urinary System CPT Code Description Auditory System 69100 Biopsy external ear 69110 Excision external ear; partial, simple repair 69140 Excision exostosis(es), external auditory canal 69145 Excision soft tissue lesion, external auditory canal 69205 Removal foreign body from external auditory canal; with general anesthesia 69222 Debridement, mastoidectomy cavity, complex (e.g., with anesthesia or more