Radial Glial Cell–Neuron Interaction Directs Axon Formation at the Opposite Side of the Neuron from the Contact Site
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
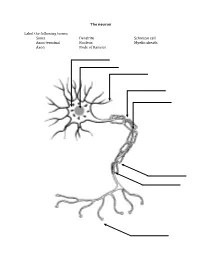
The Neuron Label the Following Terms: Soma Axon Terminal Axon Dendrite
The neuron Label the following terms: Soma Dendrite Schwaan cell Axon terminal Nucleus Myelin sheath Axon Node of Ranvier Neuron Vocabulary You must know the definitions of these terms 1. Synaptic Cleft 2. Neuron 3. Impulse 4. Sensory Neuron 5. Motor Neuron 6. Interneuron 7. Body (Soma) 8. Dendrite 9. Axon 10. Action Potential 11. Myelin Sheath (Myelin) 12. Afferent Neuron 13. Threshold 14. Neurotransmitter 15. Efferent Neurons 16. Axon Terminal 17. Stimulus 18. Refractory Period 19. Schwann 20. Nodes of Ranvier 21. Acetylcholine STEPS IN THE ACTION POTENTIAL 1. The presynaptic neuron sends neurotransmitters to postsynaptic neuron. 2. Neurotransmitters bind to receptors on the postsynaptic cell. - This action will either excite or inhibit the postsynaptic cell. - The soma becomes more positive. 3. The positive charge reaches the axon hillock. - Once the threshold of excitation is reached the neuron will fire an action potential. 4. Na+ channels open and Na+ is forced into the cell by the concentration gradient and the electrical gradient. - The neuron begins to depolarize. 5. The K+ channels open and K+ is forced out of the cell by the concentration gradient and the electrical gradient. - The neuron is depolarized. 6. The Na+ channels close at the peak of the action potential. - The neuron starts to repolarize. 7. The K+ channels close, but they close slowly and K+ leaks out. 8. The terminal buttons release neurotransmitter to the postsynaptic neuron. 9. The resting potential is overshot and the neuron falls to a -90mV (hyperpolarizes). - The neuron continues to repolarize. 10. The neuron returns to resting potential. The Synapse Label the following terms: Pre-synaptic membrane Neurotransmitters Post-synaptic membrane Synaptic cleft Vesicle Post-synaptic receptors . -

Resting and Active Properties of Pyramidal Neurons in Subiculum and CA1 of Rat Hippocampus
Resting and Active Properties of Pyramidal Neurons in Subiculum and CA1 of Rat Hippocampus NATHAN P. STAFF, HAE-YOON JUNG, TARA THIAGARAJAN, MICHAEL YAO, AND NELSON SPRUSTON Department of Neurobiology and Physiology, Institute for Neuroscience, Northwestern University, Evanston, Illinois 60208 Received 22 March 2000; accepted in final form 8 August 2000 Staff, Nathan P., Hae-Yoon Jung, Tara Thiagarajan, Michael region (Chrobak and Buzsaki 1996; Colling et al. 1998). Sub- Yao, and Nelson Spruston. Resting and active properties of pyrami- iculum has been shown to be the major output center of the dal neurons in subiculum and CA1 of rat hippocampus. J Neuro- hippocampus, to which it belongs, sending parallel projections physiol 84: 2398–2408, 2000. Action potentials are the end product of synaptic integration, a process influenced by resting and active neu- from various subicular regions to different cortical and subcor- ronal membrane properties. Diversity in these properties contributes tical areas (Naber and Witter 1998). Functionally, the principal to specialized mechanisms of synaptic integration and action potential neurons of the subiculum appear to be “place cells,” similar to firing, which are likely to be of functional significance within neural those of CA1 (Sharp and Green 1994); and in a human mag- circuits. In the hippocampus, the majority of subicular pyramidal netic resonance imaging (MRI) study, subiculum was shown to neurons fire high-frequency bursts of action potentials, whereas CA1 be an area involved in memory retrieval (Gabrieli et al. 1997). pyramidal neurons exhibit regular spiking behavior when subjected to Therefore both CA1 and subiculum have been implicated in direct somatic current injection. -

Crayfish Escape Behavior and Central Synapses
Crayfish Escape Behavior and Central Synapses. III. Electrical Junctions and Dendrite Spikes in Fast Flexor Motoneurons ROBERT S. ZUCKER Department of Biological Sciences and Program in the Neurological Sciences, Stanford University, Stanford, California 94305 THE LATERAL giant fiber is the decision fiber and fast flexor motoneurons are located in for a behavioral response in crayfish in- the dorsal neuropil of each abdominal gan- volving rapid tail flexion in response to glion. Dye injections and anatomical recon- abdominal tactile stimulation (27). Each structions of ‘identified motoneurons reveal lateral giant impulse is followed by a rapid that only those motoneurons which have tail flexion or tail flip, and when the giant dentritic branches in contact with a giant fiber does not fire in response to such stim- fiber are activated by that giant fiber (12, uli, the movement does not occur. 21). All of tl ie fast flexor motoneurons are The afferent limb of the reflex exciting excited by the ipsilateral giant; all but F4, the lateral giant has been described (28), F5 and F7 are also excited by the contra- and the habituation of the behavior has latkral giant (21 a). been explained in terms of the properties The excitation of these motoneurons, of this part of the neural circuit (29). seen as depolarizing potentials in their The properties of the neuromuscular somata, does not always reach threshold for junctions between the fast flexor motoneu- generating a spike which propagates out the rons and the phasic flexor muscles have also axon to the periphery (14). Indeed, only t,he been described (13). -

Long-Term Adult Human Brain Slice Cultures As a Model System to Study
TOOLS AND RESOURCES Long-term adult human brain slice cultures as a model system to study human CNS circuitry and disease Niklas Schwarz1, Betu¨ l Uysal1, Marc Welzer2,3, Jacqueline C Bahr1, Nikolas Layer1, Heidi Lo¨ ffler1, Kornelijus Stanaitis1, Harshad PA1, Yvonne G Weber1,4, Ulrike BS Hedrich1, Ju¨ rgen B Honegger4, Angelos Skodras2,3, Albert J Becker5, Thomas V Wuttke1,4†*, Henner Koch1†* 1Department of Neurology and Epileptology, Hertie-Institute for Clinical Brain Research, University of Tu¨ bingen, Tu¨ bingen, Germany; 2Department of Cellular Neurology, Hertie-Institute for Clinical Brain Research, University of Tu¨ bingen, Tu¨ bingen, Germany; 3German Center for Neurodegenerative Diseases (DZNE), Tu¨ bingen, Germany; 4Department of Neurosurgery, University of Tu¨ bingen, Tu¨ bingen, Germany; 5Department of Neuropathology, Section for Translational Epilepsy Research, University Bonn Medical Center, Bonn, Germany Abstract Most of our knowledge on human CNS circuitry and related disorders originates from model organisms. How well such data translate to the human CNS remains largely to be determined. Human brain slice cultures derived from neurosurgical resections may offer novel avenues to approach this translational gap. We now demonstrate robust preservation of the complex neuronal cytoarchitecture and electrophysiological properties of human pyramidal neurons *For correspondence: in long-term brain slice cultures. Further experiments delineate the optimal conditions for efficient [email protected] viral transduction of cultures, enabling ‘high throughput’ fluorescence-mediated 3D reconstruction tuebingen.de (TVW); of genetically targeted neurons at comparable quality to state-of-the-art biocytin fillings, and [email protected] demonstrate feasibility of long term live cell imaging of human cells in vitro. -

Plp-Positive Progenitor Cells Give Rise to Bergmann Glia in the Cerebellum
Citation: Cell Death and Disease (2013) 4, e546; doi:10.1038/cddis.2013.74 OPEN & 2013 Macmillan Publishers Limited All rights reserved 2041-4889/13 www.nature.com/cddis Olig2/Plp-positive progenitor cells give rise to Bergmann glia in the cerebellum S-H Chung1, F Guo2, P Jiang1, DE Pleasure2,3 and W Deng*,1,3,4 NG2 (nerve/glial antigen2)-expressing cells represent the largest population of postnatal progenitors in the central nervous system and have been classified as oligodendroglial progenitor cells, but the fate and function of these cells remain incompletely characterized. Previous studies have focused on characterizing these progenitors in the postnatal and adult subventricular zone and on analyzing the cellular and physiological properties of these cells in white and gray matter regions in the forebrain. In the present study, we examine the types of neural progeny generated by NG2 progenitors in the cerebellum by employing genetic fate mapping techniques using inducible Cre–Lox systems in vivo with two different mouse lines, the Plp-Cre-ERT2/Rosa26-EYFP and Olig2-Cre-ERT2/Rosa26-EYFP double-transgenic mice. Our data indicate that Olig2/Plp-positive NG2 cells display multipotential properties, primarily give rise to oligodendroglia but, surprisingly, also generate Bergmann glia, which are specialized glial cells in the cerebellum. The NG2 þ cells also give rise to astrocytes, but not neurons. In addition, we show that glutamate signaling is involved in distinct NG2 þ cell-fate/differentiation pathways and plays a role in the normal development of Bergmann glia. We also show an increase of cerebellar oligodendroglial lineage cells in response to hypoxic–ischemic injury, but the ability of NG2 þ cells to give rise to Bergmann glia and astrocytes remains unchanged. -

Oligodendrocytes in Development, Myelin Generation and Beyond
cells Review Oligodendrocytes in Development, Myelin Generation and Beyond Sarah Kuhn y, Laura Gritti y, Daniel Crooks and Yvonne Dombrowski * Wellcome-Wolfson Institute for Experimental Medicine, Queen’s University Belfast, Belfast BT9 7BL, UK; [email protected] (S.K.); [email protected] (L.G.); [email protected] (D.C.) * Correspondence: [email protected]; Tel.: +0044-28-9097-6127 These authors contributed equally. y Received: 15 October 2019; Accepted: 7 November 2019; Published: 12 November 2019 Abstract: Oligodendrocytes are the myelinating cells of the central nervous system (CNS) that are generated from oligodendrocyte progenitor cells (OPC). OPC are distributed throughout the CNS and represent a pool of migratory and proliferative adult progenitor cells that can differentiate into oligodendrocytes. The central function of oligodendrocytes is to generate myelin, which is an extended membrane from the cell that wraps tightly around axons. Due to this energy consuming process and the associated high metabolic turnover oligodendrocytes are vulnerable to cytotoxic and excitotoxic factors. Oligodendrocyte pathology is therefore evident in a range of disorders including multiple sclerosis, schizophrenia and Alzheimer’s disease. Deceased oligodendrocytes can be replenished from the adult OPC pool and lost myelin can be regenerated during remyelination, which can prevent axonal degeneration and can restore function. Cell population studies have recently identified novel immunomodulatory functions of oligodendrocytes, the implications of which, e.g., for diseases with primary oligodendrocyte pathology, are not yet clear. Here, we review the journey of oligodendrocytes from the embryonic stage to their role in homeostasis and their fate in disease. We will also discuss the most common models used to study oligodendrocytes and describe newly discovered functions of oligodendrocytes. -

Neuregulin 1–Erbb2 Signaling Is Required for the Establishment of Radial Glia and Their Transformation Into Astrocytes in Cerebral Cortex
Neuregulin 1–erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex Ralf S. Schmid*, Barbara McGrath*, Bridget E. Berechid†, Becky Boyles*, Mark Marchionni‡, Nenad Sˇ estan†, and Eva S. Anton*§ *University of North Carolina Neuroscience Center and Department of Cell and Molecular Physiology, University of North Carolina School of Medicine, Chapel Hill, NC 27599; †Department of Neurobiology, Yale University School of Medicine, New Haven, CT 06510; and ‡CeNes Pharamceuticals, Inc., Norwood, MA 02062 Communicated by Pasko Rakic, Yale University School of Medicine, New Haven, CT, January 27, 2003 (received for review December 12, 2002) Radial glial cells and astrocytes function to support the construction mine whether NRG-1-mediated signaling is involved in radial and maintenance, respectively, of the cerebral cortex. However, the glial cell development and differentiation in the cerebral cortex. mechanisms that determine how radial glial cells are established, We show that NRG-1 signaling, involving erbB2, may act in maintained, and transformed into astrocytes in the cerebral cortex are concert with Notch signaling to exert a critical influence in the not well understood. Here, we show that neuregulin-1 (NRG-1) exerts establishment, maintenance, and appropriate transformation of a critical role in the establishment of radial glial cells. Radial glial cell radial glial cells in cerebral cortex. generation is significantly impaired in NRG mutants, and this defect can be rescued by exogenous NRG-1. Down-regulation of expression Materials and Methods and activity of erbB2, a member of the NRG-1 receptor complex, leads Clonal Analysis to Study NRG’s Role in the Initial Establishment of to the transformation of radial glial cells into astrocytes. -

Chapter 8 Nervous System
Chapter 8 Nervous System I. Functions A. Sensory Input – stimuli interpreted as touch, taste, temperature, smell, sound, blood pressure, and body position. B. Integration – CNS processes sensory input and initiates responses categorizing into immediate response, memory, or ignore C. Homeostasis – maintains through sensory input and integration by stimulating or inhibiting other systems D. Mental Activity – consciousness, memory, thinking E. Control of Muscles & Glands – controls skeletal muscle and helps control/regulate smooth muscle, cardiac muscle, and glands II. Divisions of the Nervous system – 2 anatomical/main divisions A. CNS (Central Nervous System) – consists of the brain and spinal cord B. PNS (Peripheral Nervous System) – consists of ganglia and nerves outside the brain and spinal cord – has 2 subdivisions 1. Sensory Division (Afferent) – conducts action potentials from PNS toward the CNS (by way of the sensory neurons) for evaluation 2. Motor Division (Efferent) – conducts action potentials from CNS toward the PNS (by way of the motor neurons) creating a response from an effector organ – has 2 subdivisions a. Somatic Motor System – controls skeletal muscle only b. Autonomic System – controls/effects smooth muscle, cardiac muscle, and glands – 2 branches • Sympathetic – accelerator “fight or flight” • Parasympathetic – brake “resting and digesting” * 4 Types of Effector Organs: skeletal muscle, smooth muscle, cardiac muscle, and glands. III. Cells of the Nervous System A. Neurons – receive stimuli and transmit action potentials -

Wnt/PCP Signaling Contribution to Carcinoma Collective Cell Migration and Metastasis Kacey Vandervorst1, Courtney A
Published OnlineFirst April 5, 2019; DOI: 10.1158/0008-5472.CAN-18-2757 Cancer Review Research Wnt/PCP Signaling Contribution to Carcinoma Collective Cell Migration and Metastasis Kacey VanderVorst1, Courtney A. Dreyer1, Sara E. Konopelski2, Hyun Lee1, Hsin-Yi Henry Ho2, and Kermit L. Carraway III1 Abstract Our understanding of the cellular mechanisms governing discerned. Wnt/PCP (planar cell polarity) signaling, one of carcinoma invasiveness and metastasis has evolved dramati- the noncanonical Wnt signaling pathways, mediates collective cally over the last several years. The previous emphasis on the migratory events such as convergent extension during devel- epithelial–mesenchymal transition as a driver of the migratory opmental processes. Wnt/PCP signaling components are fre- properties of single cells has expanded with the observation quently dysregulated in solid tumors, and aberrant pathway that carcinoma cells often invade and migrate collectively as activation contributes to tumor cell migratory properties. Here adherent groups. Moreover, recent analyses suggest that cir- we summarize key studies that address the mechanisms by culating tumor cells within the vasculature often exist as which Wnt/PCP signaling mediate collective cell migration in multicellular clusters and that clusters more efficiently seed developmental and tumor contexts. We emphasize Wnt/PCP metastatic lesions than single circulating tumor cells. While component localization within migrating cells and discuss these observations point to a key role for collective cell how component asymmetry may govern the spatiotemporal migration in carcinoma metastasis, the molecular mechan- control of downstream cytoskeletal effectors to promote isms driving collective tumor cell migration remain to be collective cell motility. Introduction properties of the surrounding environment (haptotaxis or dur- otaxis; refs. -

Neuron Morphology Influences Axon Initial Segment Plasticity1,2,3
New Research Neuronal Excitability Neuron Morphology Influences Axon Initial Segment Plasticity1,2,3 Allan T. Gulledge1 and Jaime J. Bravo2 DOI:http://dx.doi.org/10.1523/ENEURO.0085-15.2016 1Department of Physiology and Neurobiology, Geisel School of Medicine at Dartmouth, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire 03756, and 2Thayer School of Engineering at Dartmouth, Hanover, New Hampshire 03755 Visual Abstract In most vertebrate neurons, action potentials are initiated in the axon initial segment (AIS), a specialized region of the axon containing a high density of voltage-gated sodium and potassium channels. It has recently been proposed that neurons use plasticity of AIS length and/or location to regulate their intrinsic excitability. Here we quantify the impact of neuron morphology on AIS plasticity using computational models of simplified and realistic somatodendritic morphologies. In small neurons (e.g., den- tate granule neurons), excitability was highest when the AIS was of intermediate length and located adjacent to the soma. Conversely, neu- rons having larger dendritic trees (e.g., pyramidal neurons) were most excitable when the AIS was longer and/or located away from the soma. For any given somatodendritic morphology, increasing dendritic mem- brane capacitance and/or conductance favored a longer and more distally located AIS. Overall, changes to AIS length, with corresponding changes in total sodium conductance, were far more effective in regulating neuron excitability than were changes in AIS location, while dendritic capacitance had a larger impact on AIS performance than did dendritic conductance. The somatodendritic influence on AIS performance reflects modest soma- to-AIS voltage attenuation combined with neuron size-dependent changes in AIS input resistance, effective membrane time constant, and isolation from somatodendritic capacitance. -

The Narrowing of Dendrite Branches Across Nodes Follows a Well-Defined Scaling Law
The narrowing of dendrite branches across nodes follows a well-defined scaling law Maijia Liaoa, Xin Liangb, and Jonathon Howarda,1 aDepartment of Molecular Biophysics and Biochemistry, Yale University, New Haven, CT 06520; and bTsinghua-Peking Joint Center for Life Sciences, School of Life Sciences, Tsinghua University, 100084 Beijing, China Edited by Yuh Nung Jan, Howard Hughes Medical Institute, University of California San Francisco, San Francisco, CA, and approved May 17, 2021 (received for review October 28, 2020) The systematic variation of diameters in branched networks has minimized. Da Vinci’s law has been reformulated as the “pipe tantalized biologists since the discovery of da Vinci’s rule for trees. model” for plants, in which a fixed cross-section of stems and Da Vinci’s rule can be formulated as a power law with exponent branches is required to support each unit amount of leaves (13). two: The square of the mother branch’s diameter is equal to the Experimental support for scaling laws, however, is scarce because sum of the squares of those of the daughters. Power laws, with of the difficulties of imaging entire branched networks and because different exponents, have been proposed for branching in circula- intrinsic anatomical variability may obscure precise laws (14). Thus, tory systems (Murray’s law with exponent 3) and in neurons (Rall’s it is an open question whether scaling laws such as Eq. 1 apply in law with exponent 3/2). The laws have been derived theoretically, biological systems. based on optimality arguments, but, for the most part, have not To quantitatively test diameter-scaling laws in neurons, it is been tested rigorously. -

Cell Polarity Ing
news and views ment of the RAFOS float speeds to results of N a e e the MODAS — Modular Ocean Data Assimi- o t r r S B th ia e ra d d t zi e e a l C R w lation System — model, which assimilates 0° . rm te Benguela in satellite altimetric measurement of sea-level undercurrent variability. m ic o f fr i Benguela C. r c There is still a great deal to learn about the a te a P e Agulhas valve, and its variation under differ- ° il W h 30 S z nt Cape t a e r r Agulhas Current ent climatic conditions. Ensuring that it is r Cauldron B u C properly represented in global ocean and cli- mate models remains a daunting challenge. South Atlantic Agulhas return current ke rrent 2 ra e Cu Agulhas retroflection But this collection of papers shows how the ° D ag 60 S ss Pa brotherhood of observers armed with new tools, aided by satellite-based remote sens- 60°W0° 60°E 120°E ing, and modellers with their increasingly realistic simulations, can take us forward. ■ Figure 1 The Agulhas system and associated flow patterns. The Agulhas Current draws water from the Arnold L. Gordon is at the Lamont–Doherty Earth Pacific Ocean through the Indonesian throughflow and Drake Passage, and from the Tasman Sea. It Observatory, Columbia University, Palisades, New abruptly turns back towards the Indian Ocean near 20° E. Here, at the Agulhas retroflection, ‘leakage’ York 10964, USA. of water occurs within an array of cyclonic (clockwise) and anticyclonic (anticlockwise) eddies that e-mail: [email protected] are injected into the vigorous stirring and mixing environment of the Cape Basin (the ‘Cape 1.