Surface Modification of Poly(Vinyl Chloride) by Physisorbed
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

II. Plasticizer-Free Polyvinyl Chloride, Plasticizer-Free Copolymers of Vinyl
This is an unofficial translation. Only the German version is binding. II. Plasticizer-free Polyvinyl Chloride, Plasticizer-free Copolymers of Vinyl Chlo- ride and Mixtures of these Polymers with other Copolymers and Chlorinated Polyolefins Containing Mainly Vinyl Chloride in the Total Mixture As of 01.01.2012 The monomers and other starting substances as well as additives used in the production of plasticizer-free polyvinyl chloride, plasticizer-free copolymers of vinyl chloride containing mainly vinyl chloride, mixtures of these polymers with other copolymers, and chlorinated polyolefins containing mainly vinyl chloride in the total mixture are subject to the requirements of the Commission Regulation (EU) No 10/2011. Otherwise, there are no objections to the use of these plastics for commodities in the sense of § 2, Para. 6, No 1 of the Food and Feed Code (Lebensmittel- und Futtermittelgesetzbuch), pro- vided they are suitable for their intended purpose and comply with the following conditions: 1. The use of monomers and other starting materials for polyethylene is subject to the stipula- tions of the Commission Regulation (EU) No 10/2011. The evaluation presented in the following refers to polymers from the following monomeric starting substances: a) Vinyl chloride b) Vinylidene chloride c) Trans-dichloroethylene d) Vinylesters of aliphatic carbonic acids C2-C18, in so far as covered by the positive list of the Commission Regulation (EU) No 10/2011 e) Esters of acrylic acid, methacrylic acid and/or maleic acid or fumaric acid with -

International Journal of Advanced Scientific and Technical Research
International journal of advanced scientific and technical research Issue 5 volume 1, January-February 2015 Available online on http://www.rspublication.com/ijst/index.html ISSN 2249-9954 Degradation Studies on Bronopol in Oral Hygiene Formulations Jampala Balaji1*, N.K.Sarthchandra Prakash2, 3 Krishan Manaral R&D Department, The Himalaya Drug Company, Makali, Bangalore, India-562162. ABSTRACT This study investigated the decomposition of Bronopol and its degradation products how they produce Nitrosamines in oral hygiene products. Based on this study Bronopol was degradable in oral hygiene products which are containing aqueous medium, higher pH values (4 to10) and which are presented in higher temperatures and then gives formaldehyde, and nitro compounds. These nitro compounds are acting as a nitrosating agent and react with secondary amines then give Nitrosamines in the products. The degradation study was done by using determination of mass by LCMS and determination of formaldehyde content by Head-Space GC. These studies strongly demonstrate the Bronopol was degradable in aqueous medium and give formaldehyde and nitro compounds. Bronopol was not acting itself as a nitrosating agent and its degradation products act as a nitrosating agent. The products which are containing the secondary amines and the nitrosating agent then the Nitrosamines are formed in that product. Keywords: Bronopol, Degradation, Nitrosamines, Formaldehyde, Oral hygiene products. INTRODUCTION Bronopol is an organic compound also called as a 2-bromo-2-nitropropane-1, 3- diol (Molecular formula: C(Br)(NO2)(CH2OH)2). In cosmetics Bronopol is used as an antibacterial preservative to prevent spoilage due to microbial contamination [1]. Bronopol structure was shown in fig1. -

Using an Inhibitor to Prevent Plasticizer Migration from Polyurethane Matrix to EPDM Based Substrate Rezaei-Vahidian Hadi, Farajpour Tohid, Abdollahi Mahdi
Using an Inhibitor to Prevent Plasticizer Migration from Polyurethane Matrix to EPDM Based Substrate Rezaei-Vahidian Hadi, Farajpour Tohid, Abdollahi Mahdi Cite this article as: Rezaei-Vahidian Hadi, Farajpour Tohid, Abdollahi Mahdi. Using an Inhibitor to Prevent Plasticizer Migration from Polyurethane Matrix to EPDM Based Substrate[J]. Chinese J. Polym. Sci, 2019, 37(7): 681-686. doi: 10.1007/s10118-019-2251-y View online: https://doi.org/10.1007/s10118-019-2251-y Articles you may be interested in Antistatic PVC-graphene Composite through Plasticizer-mediated Exfoliation of Graphite Chinese J. Polym. Sci. 2018, 36(12): 1361 https://doi.org/10.1007/s10118-018-2160-5 SYNTHESIS OF POLYURETHANE MODIFIED BISMALEIMIDE (UBMI) AND POLYURETHANE-IMIDE ELASTOMER Chinese J. Polym. Sci. 2008, 26(1): 117 Synthesis and Properties of Reversible Disulfide Bond-based Self-healing Polyurethane with Triple Shape Memory Properties Chinese J. Polym. Sci. 2019, 37(11): 1119 https://doi.org/10.1007/s10118-019-2268-2 增塑剂对碱木质素/HDPE复合材料性能影响研究 The Effect of Plasticizer on the Properties of Alkali Lignin/HDPE Composites 高分子学报. 2014(2): 210 https://doi.org/10.3724/SP.J.1105.2014.13204 泊肃叶流中环形高分子的迁移行为及与线性高分子的差异 Migration of Ring Polymers in Poiseuille Flow and Comparison with Linear Polymers 高分子学报. 2019, 50(11): 1229 https://doi.org/10.11777/j.issn1000-3304.2019.19074 聚醚硅油表面迁移行为对聚苯乙烯表面极性的影响 THE IMPROVEMENT OF POLARITY OF POLYSTYRENE SURFACE BY THE SELECTIVE SURFACE MIGRATION OF POLYETHER SILICOME OIL 高分子学报. 2007(2): 165 Chinese Journal of POLYMER SCIENCE ARTICLE https://doi.org/10.1007/s10118-019-2251-y -

Recent Attempts in the Design of Efficient PVC Plasticizers With
materials Review Recent Attempts in the Design of Efficient PVC Plasticizers with Reduced Migration Joanna Czogała 1,2,3,* , Ewa Pankalla 2 and Roman Turczyn 1,* 1 Department of Physical Chemistry and Technology of Polymers, Silesian University of Technology, Strzody 9, 44-100 Gliwice, Poland 2 Research and Innovation Department, Grupa Azoty Zakłady Azotowe K˛edzierzynS.A., Mostowa 30A, 47-220 K˛edzierzyn-Ko´zle,Poland; [email protected] 3 Joint Doctoral School, Silesian University of Technology, Akademicka 2A, 44-100 Gliwice, Poland * Correspondence: [email protected] (J.C.); [email protected] (R.T.) Abstract: This paper reviews the current trends in replacing commonly used plasticizers in poly(vinyl chloride), PVC, formulations by new compounds with reduced migration, leading to the enhance- ment in mechanical properties and better plasticizing efficiency. Novel plasticizers have been divided into three groups depending on the replacement strategy, i.e., total replacement, partial replacement, and internal plasticizers. Chemical and physical properties of PVC formulations containing a wide range of plasticizers have been compared, allowing observance of the improvements in polymer per- formance in comparison to PVC plasticized with conventionally applied bis(2-ethylhexyl) phthalate, di-n-octyl phthalate, bis(2-ethylhexyl) terephthalate and di-n-octyl terephthalate. Among a variety of newly developed plasticizers, we have indicated those presenting excellent migration resistance and advantageous mechanical properties, as well as those derived from natural sources. A separate chapter has been dedicated to the description of a synergistic effect of a mixture of two plasticizers, primary and secondary, that benefits in migration suppression when secondary plasticizer is added to PVC blend. -

Cross Linked Sulphonated Poly(Ether Ether Ketone) for the Development of Polymer Electrolyte Membrane Fuel Cell
Cross Linked Sulphonated Poly(ether ether ketone) for the Development of Polymer Electrolyte Membrane Fuel Cell Abdul Ghaffar Al Lafi, MSc. A Thesis Submitted for the Degree of Doctor of Philosophy The School of Metallurgy and Materials, The College of Engineering and Physical Sciences, The University of Birmingham, Birmingham, UK. September 2009 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. Synopsis Ion irradiation has been investigated as a route for the preparation of mechanically stable and highly durable cross linked sulphonated PEEK for fuel cell application. Ion irradiation was carried out using the University of Birmingham’s Scanditronix MC-40 Cyclotron operating at 11.7 MeV for H+ and 30 MeV for He2+ and the irradiated materials were characterized focusing on structural, thermal, morphological as well as dielectrical properties. Alterations produced in the molecular structure of amorphous PEEK by ion irradiation have been interpreted as due to chain scission and formation of cross links, as confirmed by sol-gel analysis in MSA using the well known Charlesby–Pinner equation. The thermal decomposition of irradiated PEEK was similar to that of untreated PEEK in that it occurred by a random chain scission process. -

Cosmetic Ingredients/Material Prohibited/Restricted by FDA (14 Total, Through November, 2011)
Cosmetic ingredients/material prohibited/restricted by FDA (14 total, through November, 2011). For the most part, FDA regulatory action to ban use of an ingredient in cosmetics means that ingredient is exempt from CIR review. In the case of Methylene Chloride, CIR=s safety assessment that preceded FDA=s regulation is now superceded. In the case of Tallow and tallow derivatives, the FDA identification of prohibited cattle material does not ban Tallow and tallow derivatives use in cosmetics. In the case of Trichloroethane, the EPA ban (except for essential uses) and the FDA determination of non-essential use appears to relate to aerosol cosmetic uses only. Ingredient No. CIR Conclusion FDA Regulation 1 Bithionol Exempt from CIR review Cosmetics containing bithionol.are deemed to be adulterated because of FDA under section 601(a) of the Federal Food, Drug, and Cosmetic regulation. Act (21CFR'700.11) 1 Chlorofluorocarbon propellants Exempt from CIR review FDA has prohibited the use of Chlorofluorocarbon propellants in because of FDA cosmetic products with self-pressurized containers regulation. (21CFR'700.23) 1 Chloroform Exempt from CIR review FDA has prohibited the use of Chloroform, except if present in because of FDA residual amounts from its use as a processing solvent during regulation manufacture, or as a byproduct from the synthesis of an ingredient (21CFR'700.18) 4 Halogenated salicylanilides Exempt from CIR review FDA has prohibited (21CFR'700.15) the use of four because of FDA halogenated salicylanilides: regulation tribromsalan (TBS,3,4 Œ,5Btribromosalicylanilide), dibromsalan (DBS,4 Œ5Bdibromosalicylanilide), metabromsalan (MBS, 3,5 B dibromosalicylanilide) and 3,3 Œ,4,5 ŒB tetrachlorosalicylanilide (TCSA) 1 Hexachlorophene Exempt from CIR review Not allowed as a preservative in cosmetics where normal use because of FDA may be applied to mucous membranes or which are intended to regulation (CIR has be used on mucous membranes. -

High Performance Ester Plasticizers
High Performance Ester Plasticizers Abstract Traditional elastomer polymers, such as nitrile, polychloroprene, chlorinated polyethylene and chlorosulfonated polyethylene, have for years used moderate- to low- performance ester plasticizers. However, longevity requirements for rubber articles made from these elastomers have created a need for higher-performance ester plasticizers. With the increasing high-temperature demands required by automotive, other elastomers such as acrylic, high-saturated nitrile, epichlorohydrin and ethylene propylene diene monomer EPDM are replacing the more traditional elastomers. Plasticizers commonly used for the traditional and the high-temperature polymers are extractable, incompatible or too volatile. This paper provides information on plasticizers that are designed for traditional elastomers and high-performance polymers. The test data will include heat aging, extraction by hydrocarbons and low-temperature as molded after aging. The information provided indicates that the permanence of the plasticizer after these various agings is the key to the retention of physical properties. Introduction End uses for elastomer compounds are quite diverse, but they can be loosely categorized as being either general performance or higher performance applications. Each of these performance categories requires a different set of considerations in terms of compounding with ester plasticizers. An ester plasticizer, in its simplest concept, is a high-boiling organic solvent that when added to an elastomeric polymer reduces stiffness and permits easier processing.1 For general performance applications, compounders require moderate performance in several areas without particular emphasis on any one. Some general performance ester plasticizers used in the marketplace today are DOA, DIDA, DIDP, DOP, DINP and other phthalates and adipates made from straight-chain alcohols of 7–11 carbons in length. -

Simultaneous Determination of Isothiazolinones
pharmaceuticals Article Simultaneous Determination of Isothiazolinones and Parabens in Cosmetic Products Using Solid-Phase Extraction and Ultra-High Performance Liquid Chromatography/Diode Array Detector Hazim Mohammed Ali 1,2 , Ibrahim Hotan Alsohaimi 1 , Mohammad Rizwan Khan 3,* and Mohammad Azam 3 1 Department of Chemistry, College of Science, Jouf University, Sakaka 2014, Saudi Arabia; [email protected] (H.M.A.); [email protected] (I.H.A.) 2 Forensic Chemistry Department, Forensic Medicine Authority, Cairo 11441, Egypt 3 Department of Chemistry, College of Science, King Saud University, Riyadh 11451, Saudi Arabia; [email protected] * Correspondence: [email protected]; Tel.: +966-114-674-198; Fax: +966-114-675-992 Received: 22 October 2020; Accepted: 20 November 2020; Published: 22 November 2020 Abstract: Isothiazolinones methylisothiazolinone (MI) and methylchloroisothiazolinone (MCI), and parabens methylparaben (MP), ethylparaben (EP), propylparaben (PP) and butylparaben (BP) are the most common synthetic preservatives. They are all known to be potential skin allergens that lead to contact dermatitis. Thus, the identification of these unsafe chemicals in cosmetic products is of high importance. In the present study, solid-phase extraction (SPE) based on HyperSep reversed-phase C8/benzene sulfonic acid ion exchanger (HyperSep C8/BSAIE) and Sep-Pak C18 sorbents, and ultra-high performance liquid chromatography/diode array detector (UHPLC/DAD) were optimized for the simultaneous determination of MI, MCI, MP,EP,PP and BP in cosmetic products. HyperSep C8/BSAIE and UHPLC/DAD with the eluting solvent mixture (acetonitrile/methanol, 2:1, v/v) and detection wavelength (255 nm) were found to be the optimal conditions, respectively. -
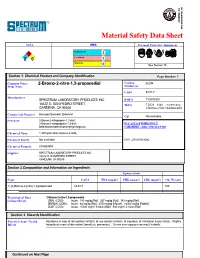
Material Safety Data Sheet
Material Safety Data Sheet NFPA HMIS Personal Protective Equipment Health Hazard 1 2 2 0 Fire Hazard 2 Reactivity 0 See Section 15. Section 1. Chemical Product and Company Identification Page Number: 1 Common Name/ 2-Bromo-2-nitro-1,3-propanediol Catalog B2294 Trade Name Number(s). CAS# 52-51-7 Manufacturer SPECTRUM LABORATORY PRODUCTS INC. RTECS TY3385000 14422 S. SAN PEDRO STREET TSCA TSCA 8(b) inventory: GARDENA, CA 90248 2-Bromo-2-nitro-1,3-propanediol Commercial Name(s) Bronopol;Bronidiol; Bronocot CI# Not available. Synonym 2-Bromo-2-nitropropan-1,3-diol; 2-Bromo-2-nitropropane-1,3-diol; IN CASE OF EMERGENCY beta-Bromo-beta-nitrotrimethyleneglycol CHEMTREC (24hr) 800-424-9300 Chemical Name 1,3-Propanediol,2-bromo-2-nitro- Chemical Family Not available. CALL (310) 516-8000 Chemical Formula C3H6BrNO4 Supplier SPECTRUM LABORATORY PRODUCTS INC. 14422 S. SAN PEDRO STREET GARDENA, CA 90248 Section 2.Composition and Information on Ingredients Exposure Limits Name CAS # TWA (mg/m 3) STEL (mg/m 3) CEIL (mg/m 3) % by Weight 1) {2-}Bromo{-2-}nitro{-1,3-}propanediol 52-51-7 100 Toxicological Data 2-Bromo-2-nitro-1,3-propanediol : on Ingredients ORAL (LD50): Acute: 180 mg/kg [Rat]. 267 mg/kg [Rat]. 342 mg/kg [Rat]. DERMAL (LD50): Acute: 64 mg/kg [Rat]. 4750 mg/kg [Mouse]. >2000 mg/kg [Rabbit]. DUST (LC50): Acute: >5000 mg/m 3 6 hours [Rat]. 800 mg/m 3 4 hours [Rat]. Section 3. Hazards Identification Potential Acute Health Hazardous in case of skin contact (irritant), of eye contact (irritant), of ingestion, of inhalation (lung irritant). -

Efficacy of Continuous Exposure to Low Doses of Bronopol (Pycezetm) On
DEVELOPING STRATEGIES FOR THE CONTROL OF ICHTHYOPHTHIRIUS MULTIFILIIS FOUQUET, 1876 (CILIOPHORA) DECEMBER 2010 SARA M. PICÓN CAMACHO (BSC, MSC) A THESIS SUBMITTED FOR THE DEGREE OF DOCTOR OF PHILOSOPHY INSTITUTE OF AQUACULTURE UNIVERSITY OF STIRLING I To Eric Leclercq, for your love and support. Thank you for believing in me II Sara Picón Camacho DECLARATION This thesis has been composed in its entirety by the candidate. Except where specifically acknowledged, the work described in this thesis has been conducted independently and has not been submitted for any other degree. CANDIDATE NAME: SARA PICON CAMACHO SIGNATURE: DATE: 20/ 05/2011 SUPERVISOR NAME: ANDREW P. SHINN SIGNATURE: DATE: 20/05/2011 III Sara Picón Camacho ABSTRACT The intensification of freshwater aquaculture worldwide has facilitated the propagation of the parasitic ciliate protozoan Ichthyophthirius multifiliis Fouquet, 1876 commonly known as “fish white spot” or “Ich”. Ichthyophthirius multifiliis infections lead to high mortalities, generating significant economic losses in most cultured freshwater fish species worldwide. Until recently, malachite green was the chemical treatment traditionally used to control I. multifiliis infections. Its reclassification as carcinogenic to humans and its subsequent ban for use in food fish has left the industry without any suitable treatments. Currently, in-bath formaldehyde and sodium chloride treatments are the most common option used in farm systems to control I. multifliis infections. Given their low efficacy, however, they are not considered as sustainable long–term options. There is, therefore, an urgent necessity to find efficacious alternatives for controlling I. multifliis infections. The general aim of this research project was to improve the management of I. -

Bronopol: Reregistration Eligibility Decision (RED)
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C. 20460 OFFICE OF PREVENTION, PESTICIDES AND TOXIC SUBSTANCES CERTIFIED MAIL Dear Registrant: I am pleased to announce that the Environmental Protection Agency has completed its reregistration eligibility review and decisions on the pesticide chemical case bronopol. The enclosed Reregistration Eligibility Decision (RED) contains the Agency's evaluation of the data base of these chemicals, its conclusions of the potential human health and environmental risks of the current product uses, and its decisions and conditions under which these uses and products will be eligible for reregistration. The RED includes the data and labeling requirements for products for reregistration. It may also include requirements for additional data (generic) on the active ingredients to confirm the risk assessments. To assist you with a proper response, read the enclosed document entitled "Summary of Instructions for Responding to the RED". This summary also refers to other enclosed documents which include further instructions. You must follow all instructions and submit complete and timely responses. The first set of required responses are due 90 days from the date of this letter. The second set of required responses are due 8 months from the date of this letter. Complete and timely responses will avoid the Agency taking the enforcement action of suspension against your products. If you have questions on the product specific data requirements or wish to meet with the Agency, please contact the Special Review and Reregistration Division representative Frank Rubis (703) 308-8184. Address any questions on required generic data to the Special Review and Reregistration Division representative Ron Kendall (703) 308-8068. -

Microporous Membranes from Poly\Ether-Etherketone\-Type
Europa,schesP_ MM M M M M MINI M MM I M I M J European Patent Office a At* w%n © Publication number: 0 492 446 B1 Office europeen, desJ brevets © EUROPEAN PATENT SPECIFICATION © Date of publication of patent specification: 29.11.95 © Int. CI.6: B01 D 71/52, B01D 67/00, C08J 9/26 © Application number: 91121801.4 @ Date of filing: 19.12.91 The file contains technical information submitted after the application was filed and not included in this specification Mlcroporous membranes from poly(ether-etherketone)-type polymers and low melting point crystalllzable polymers, and a process for making the same. ® Priority: 21.12.90 US 633600 California 94526 (US) Inventor: Beck, H. Nelson @ Date of publication of application: 390 La Vista Road 01.07.92 Bulletin 92/27 Walnut Creek, California 94598 (US) © Publication of the grant of the patent: Inventor: Lundgard, Richard A. 29.11.95 Bulletin 95/48 2441 Whltetall Drive Antloch, © Designated Contracting States: California 94509 (US) DE DK FR GB IT NL Inventor: Wan, Hawk S. 4627 Fleldcrest Way © References cited: Antloch, EP-A- 0 297 744 California 94509 (US) EP-A- 0 337 626 Inventor: Kawamoto, Jlro EP-A- 0 409 416 2348 Benham Court EP-A- 0 417 908 Walnut Creek, California 94596 (US) © Proprietor: THE DOW CHEMICAL COMPANY Inventor: Sonnenscheln, Mark F. 2030 Dow Center, 3512 Blythe Drive Abbott Road Antloch, Midland, California 94509 (US) 00 Michigan 48640 (US) CO @ Inventor: Mahoney, Robert D. 240 Daylight Palace Danville, CM Oi Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted.