Bronopol: Reregistration Eligibility Decision (RED)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

International Journal of Advanced Scientific and Technical Research
International journal of advanced scientific and technical research Issue 5 volume 1, January-February 2015 Available online on http://www.rspublication.com/ijst/index.html ISSN 2249-9954 Degradation Studies on Bronopol in Oral Hygiene Formulations Jampala Balaji1*, N.K.Sarthchandra Prakash2, 3 Krishan Manaral R&D Department, The Himalaya Drug Company, Makali, Bangalore, India-562162. ABSTRACT This study investigated the decomposition of Bronopol and its degradation products how they produce Nitrosamines in oral hygiene products. Based on this study Bronopol was degradable in oral hygiene products which are containing aqueous medium, higher pH values (4 to10) and which are presented in higher temperatures and then gives formaldehyde, and nitro compounds. These nitro compounds are acting as a nitrosating agent and react with secondary amines then give Nitrosamines in the products. The degradation study was done by using determination of mass by LCMS and determination of formaldehyde content by Head-Space GC. These studies strongly demonstrate the Bronopol was degradable in aqueous medium and give formaldehyde and nitro compounds. Bronopol was not acting itself as a nitrosating agent and its degradation products act as a nitrosating agent. The products which are containing the secondary amines and the nitrosating agent then the Nitrosamines are formed in that product. Keywords: Bronopol, Degradation, Nitrosamines, Formaldehyde, Oral hygiene products. INTRODUCTION Bronopol is an organic compound also called as a 2-bromo-2-nitropropane-1, 3- diol (Molecular formula: C(Br)(NO2)(CH2OH)2). In cosmetics Bronopol is used as an antibacterial preservative to prevent spoilage due to microbial contamination [1]. Bronopol structure was shown in fig1. -

Cosmetic Ingredients/Material Prohibited/Restricted by FDA (14 Total, Through November, 2011)
Cosmetic ingredients/material prohibited/restricted by FDA (14 total, through November, 2011). For the most part, FDA regulatory action to ban use of an ingredient in cosmetics means that ingredient is exempt from CIR review. In the case of Methylene Chloride, CIR=s safety assessment that preceded FDA=s regulation is now superceded. In the case of Tallow and tallow derivatives, the FDA identification of prohibited cattle material does not ban Tallow and tallow derivatives use in cosmetics. In the case of Trichloroethane, the EPA ban (except for essential uses) and the FDA determination of non-essential use appears to relate to aerosol cosmetic uses only. Ingredient No. CIR Conclusion FDA Regulation 1 Bithionol Exempt from CIR review Cosmetics containing bithionol.are deemed to be adulterated because of FDA under section 601(a) of the Federal Food, Drug, and Cosmetic regulation. Act (21CFR'700.11) 1 Chlorofluorocarbon propellants Exempt from CIR review FDA has prohibited the use of Chlorofluorocarbon propellants in because of FDA cosmetic products with self-pressurized containers regulation. (21CFR'700.23) 1 Chloroform Exempt from CIR review FDA has prohibited the use of Chloroform, except if present in because of FDA residual amounts from its use as a processing solvent during regulation manufacture, or as a byproduct from the synthesis of an ingredient (21CFR'700.18) 4 Halogenated salicylanilides Exempt from CIR review FDA has prohibited (21CFR'700.15) the use of four because of FDA halogenated salicylanilides: regulation tribromsalan (TBS,3,4 Œ,5Btribromosalicylanilide), dibromsalan (DBS,4 Œ5Bdibromosalicylanilide), metabromsalan (MBS, 3,5 B dibromosalicylanilide) and 3,3 Œ,4,5 ŒB tetrachlorosalicylanilide (TCSA) 1 Hexachlorophene Exempt from CIR review Not allowed as a preservative in cosmetics where normal use because of FDA may be applied to mucous membranes or which are intended to regulation (CIR has be used on mucous membranes. -

Simultaneous Determination of Isothiazolinones
pharmaceuticals Article Simultaneous Determination of Isothiazolinones and Parabens in Cosmetic Products Using Solid-Phase Extraction and Ultra-High Performance Liquid Chromatography/Diode Array Detector Hazim Mohammed Ali 1,2 , Ibrahim Hotan Alsohaimi 1 , Mohammad Rizwan Khan 3,* and Mohammad Azam 3 1 Department of Chemistry, College of Science, Jouf University, Sakaka 2014, Saudi Arabia; [email protected] (H.M.A.); [email protected] (I.H.A.) 2 Forensic Chemistry Department, Forensic Medicine Authority, Cairo 11441, Egypt 3 Department of Chemistry, College of Science, King Saud University, Riyadh 11451, Saudi Arabia; [email protected] * Correspondence: [email protected]; Tel.: +966-114-674-198; Fax: +966-114-675-992 Received: 22 October 2020; Accepted: 20 November 2020; Published: 22 November 2020 Abstract: Isothiazolinones methylisothiazolinone (MI) and methylchloroisothiazolinone (MCI), and parabens methylparaben (MP), ethylparaben (EP), propylparaben (PP) and butylparaben (BP) are the most common synthetic preservatives. They are all known to be potential skin allergens that lead to contact dermatitis. Thus, the identification of these unsafe chemicals in cosmetic products is of high importance. In the present study, solid-phase extraction (SPE) based on HyperSep reversed-phase C8/benzene sulfonic acid ion exchanger (HyperSep C8/BSAIE) and Sep-Pak C18 sorbents, and ultra-high performance liquid chromatography/diode array detector (UHPLC/DAD) were optimized for the simultaneous determination of MI, MCI, MP,EP,PP and BP in cosmetic products. HyperSep C8/BSAIE and UHPLC/DAD with the eluting solvent mixture (acetonitrile/methanol, 2:1, v/v) and detection wavelength (255 nm) were found to be the optimal conditions, respectively. -
Material Safety Data Sheet
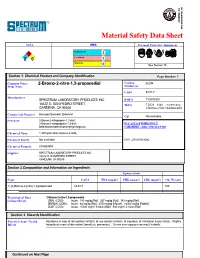
Material Safety Data Sheet NFPA HMIS Personal Protective Equipment Health Hazard 1 2 2 0 Fire Hazard 2 Reactivity 0 See Section 15. Section 1. Chemical Product and Company Identification Page Number: 1 Common Name/ 2-Bromo-2-nitro-1,3-propanediol Catalog B2294 Trade Name Number(s). CAS# 52-51-7 Manufacturer SPECTRUM LABORATORY PRODUCTS INC. RTECS TY3385000 14422 S. SAN PEDRO STREET TSCA TSCA 8(b) inventory: GARDENA, CA 90248 2-Bromo-2-nitro-1,3-propanediol Commercial Name(s) Bronopol;Bronidiol; Bronocot CI# Not available. Synonym 2-Bromo-2-nitropropan-1,3-diol; 2-Bromo-2-nitropropane-1,3-diol; IN CASE OF EMERGENCY beta-Bromo-beta-nitrotrimethyleneglycol CHEMTREC (24hr) 800-424-9300 Chemical Name 1,3-Propanediol,2-bromo-2-nitro- Chemical Family Not available. CALL (310) 516-8000 Chemical Formula C3H6BrNO4 Supplier SPECTRUM LABORATORY PRODUCTS INC. 14422 S. SAN PEDRO STREET GARDENA, CA 90248 Section 2.Composition and Information on Ingredients Exposure Limits Name CAS # TWA (mg/m 3) STEL (mg/m 3) CEIL (mg/m 3) % by Weight 1) {2-}Bromo{-2-}nitro{-1,3-}propanediol 52-51-7 100 Toxicological Data 2-Bromo-2-nitro-1,3-propanediol : on Ingredients ORAL (LD50): Acute: 180 mg/kg [Rat]. 267 mg/kg [Rat]. 342 mg/kg [Rat]. DERMAL (LD50): Acute: 64 mg/kg [Rat]. 4750 mg/kg [Mouse]. >2000 mg/kg [Rabbit]. DUST (LC50): Acute: >5000 mg/m 3 6 hours [Rat]. 800 mg/m 3 4 hours [Rat]. Section 3. Hazards Identification Potential Acute Health Hazardous in case of skin contact (irritant), of eye contact (irritant), of ingestion, of inhalation (lung irritant). -

Efficacy of Continuous Exposure to Low Doses of Bronopol (Pycezetm) On
DEVELOPING STRATEGIES FOR THE CONTROL OF ICHTHYOPHTHIRIUS MULTIFILIIS FOUQUET, 1876 (CILIOPHORA) DECEMBER 2010 SARA M. PICÓN CAMACHO (BSC, MSC) A THESIS SUBMITTED FOR THE DEGREE OF DOCTOR OF PHILOSOPHY INSTITUTE OF AQUACULTURE UNIVERSITY OF STIRLING I To Eric Leclercq, for your love and support. Thank you for believing in me II Sara Picón Camacho DECLARATION This thesis has been composed in its entirety by the candidate. Except where specifically acknowledged, the work described in this thesis has been conducted independently and has not been submitted for any other degree. CANDIDATE NAME: SARA PICON CAMACHO SIGNATURE: DATE: 20/ 05/2011 SUPERVISOR NAME: ANDREW P. SHINN SIGNATURE: DATE: 20/05/2011 III Sara Picón Camacho ABSTRACT The intensification of freshwater aquaculture worldwide has facilitated the propagation of the parasitic ciliate protozoan Ichthyophthirius multifiliis Fouquet, 1876 commonly known as “fish white spot” or “Ich”. Ichthyophthirius multifiliis infections lead to high mortalities, generating significant economic losses in most cultured freshwater fish species worldwide. Until recently, malachite green was the chemical treatment traditionally used to control I. multifiliis infections. Its reclassification as carcinogenic to humans and its subsequent ban for use in food fish has left the industry without any suitable treatments. Currently, in-bath formaldehyde and sodium chloride treatments are the most common option used in farm systems to control I. multifliis infections. Given their low efficacy, however, they are not considered as sustainable long–term options. There is, therefore, an urgent necessity to find efficacious alternatives for controlling I. multifliis infections. The general aim of this research project was to improve the management of I. -

Surface Modification of Poly(Vinyl Chloride) by Physisorbed
SURFACE MODIFICATION OF POLY(VINYL CHLORIDE) BY PHYSISORBED FREE RADICAL INITIATION FOR REDUCED PLASTICIZER MIGRATION AND ANTIMICROBIAL PROPERTIES A Dissertation Presented to The Graduate Faculty of The University of Akron In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy Kathryn McGinty December, 2008 SURFACE MODIFICATION OF POLY(VINYL CHLORIDE) BY PHYSISORBED FREE RADICAL INITIATION FOR REDUCED PLASTICIZER MIGRATION AND ANTIMICROBIAL PROPERTIES Kathryn McGinty Dissertation Approved: Accepted: Advisor Department Chair Dr. Roderic P. Quirk Dr. Ali Dhinojwala Committee Member Dean of the College Dr. Scott Collins Dr. Stephen Z.D. Cheng Committee Member Dean of the Graduate School Dr. Ali Dhinojwala Dr. George R. Newkome Committee Member Date Dr. Judit Puskas Committee Member Dr. Mark D. Soucek ii ABSTRACT Poly(vinyl chloride), PVC, is a particularly important commodity polymer that accounts for an annual world-wide production of 26 million tons. It is used frequently in the medical field as blood storage bags, endotracheal and dialysis tubing and intravenous catheters. Common plasticizers, namely di(2-ethylhexyl) phthalate (DEHP), are added to PVC to improve the processability and flexibility by lowering the glass transition temperature. However, most phthalate plasticizers are potential carcinogens. There has been extensive research on PVC with surface coatings to improve biocompatibility, surface crosslinking to create a barrier to the plasticizer leaching and surface grafting of hydrophilic polymers for both biocompatibility and reduced plasticizer migration. A novel surface grafting technique is the grafting of hydrophilic monomers by physisorbed free radical initiators. This modification method can be applied to PVC to attach vinyl hydrophilic monomers by the “grafting from” method. -

The Release of Formaldehyde Upon Decomposition of 2-Bromo-2
488 Journal of Health Science, 54(4) 488–492 (2008) The Release of Formaldehyde INTRODUCTION upon Decomposition of Antibacterial preservatives are widely used in 2-Bromo-2-nitropropan-1,3-diol cosmetics to prevent spoilage due to microbial (Bronopol) contamination. Several of these preservatives are known as formaldehyde (FA)-releasing com- pounds1–4) that release FA through decomposi- ∗ Keiji Kajimura, Takaomi Tagami, tion or degradation. 2-Bromo-2-nitropropan-1,3- Takeo Yamamoto, and Shozo Iwagami diol (bronopol: BP), one such substance, is ac- tive against a broad spectrum of bacteria, including Osaka Prefectural Institute of Public Health, 1–3–69 Gram-negative species that are resistant to many an- Nakamichi, Higashinari-ku, Osaka 537–0025, Japan tibacterial agents.5–7) In Japan, BP cannot be used (Received April 22, 2008; Accepted May 14, 2008; in cosmetics; however, in the U.S.A. and many Published online May 26, 2008) Western countries, this application is approved by law.8) We investigated the release of formaldehyde (FA) In recent years, with the rapid development of upon the decomposition of 2-bromo-2-nitropropan- information systems such as the World Wide Web, it 1,3-diol (Bronopol: BP), an FA-releasing substance has become easy to purchase cosmetics from over- widely used as an antimicrobial preservative in cos- seas. In addition, Japanese traveling abroad have metics and toiletries in many Western countries. The begun buying large amounts of cosmetics in other decomposition process producing FA mainly depends countries.9) BP, an FA-releasing substance, may be on temperature and pH. Notably, the pH of the BP- in these cosmetics. -

Potential Chemical Contaminants in the Marine Environment
Potential chemical contaminants in the marine environment An overview of main contaminant lists Victoria Tornero, Georg Hanke 2017 EUR 28925 EN This publication is a Technical report by the Joint Research Centre (JRC), the European Commission’s science and knowledge service. It aims to provide evidence-based scientific support to the European policymaking process. The scientific output expressed does not imply a policy position of the European Commission. Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use that might be made of this publication. Contact information Name: Victoria Tornero Address: European Commission Joint Research Centre, Directorate D Sustainable Resources, Water and Marine Resources Unit, Via Enrico Fermi 2749, I-21027 Ispra (VA) Email: [email protected] Tel.: +39-0332-785984 JRC Science Hub https://ec.europa.eu/jrc JRC 108964 EUR 28925 EN PDF ISBN 978-92-79-77045-6 ISSN 1831-9424 doi:10.2760/337288 Luxembourg: Publications Office of the European Union, 2017 © European Union, 2017 The reuse of the document is authorised, provided the source is acknowledged and the original meaning or message of the texts are not distorted. The European Commission shall not be held liable for any consequences stemming from the reuse. How to cite this report: Tornero V, Hanke G. Potential chemical contaminants in the marine environment: An overview of main contaminant lists. ISBN 978-92-79-77045-6, EUR 28925, doi:10.2760/337288 All images © European Union 2017 Contents Acknowledgements ................................................................................................ 1 Abstract ............................................................................................................... 2 1 Introduction ...................................................................................................... 3 2 Compilation of substances of environmental concern ............................................. -

BASF Biocides
Specialty Chemicals by BASF BASF Biocides Industrial product preservation PAGE 2 BASF Biocides - protecting the world The biocide business for Specialty Chemicals, BASF AG BASF is one of the world’s leading suppliers of How we work for you specialty biocides. We create products that make life safer, simpler and more comfortable for BASF’s biocides are sold through BASF’s millions of people worldwide. established network of sales offices worldwide. In this way, we aim to offer valuable local support Our high performance biocides are used in every and the highest standards of customer excellence. aspect of life. From toiletries, to air-conditioners used in both industry and home, from Iceland to Working with BASF provides access to many Equador our technology spans the globe in essential regulatory approvals. For example our preservation and disinfection. We help to protect EPA registrations allow products and customer the lives of millions of people across the world formulations to be sold in the USA and our every day. approvals ensure supply to the paper industry in the Nordic area. We are also well positioned to Through our product range, marketing expertise, support our actives under the European Biocidal technical and regulatory knowledge, BASF’s Products Directive. innovative biocides approach positions the business at the very forefront of antimicrobial We believe in long-term commitment to our technology. The marketing, technical and research products and customers and this is reflected in and development headquarters for BASF’s our substantial registrations and the data required specialty biocide business is located in to obtain them. -
POLYMER ADD-BRONOPOL Technical Datasheet
POLYMER ADDITIVES ANTIMICROBIAL POLYMER ADD-BRONOPOL Technical Datasheet Product Information: POLYMER ADD-BRONOPOL is an excellent preservative for both leaveon and rinse off products such as creams, lotions, shampoos, rinses, wipes and towelettes. It is synergistic with other preservatives such as Isothiazolinones, Parabens and Iodopropynyl Butylcarbamate (IPBC). It is poorly soluble in non-polar solvent but shows a high affinity for polar organic solvent. Chemical Name 2-BROMO-2-NITRO-1,3-PROPANEDIOL (BRONOPOL) Grade Name POLYMER ADD-BRONOPOL CAS No. 52-51-7 EINECS No. 200-143-0 Molecular Formula C3H6BrNO4 Synonyms 2-Bromo-2-nitro-1,3-propanediol 1,3-Propanediol, 2-bromo-2-nitro 2-Bromo-2-nitropropane-1,3-diol TEST SPECIFICATION METHOD Appearance Form: Crystalline Powder Visual Colour: White Purity 99 % Min. By HPLC Melting Range 126 - 131 °C Melting point apparatus (open capillary tube method) Moisture Content 0.5 % Max. KF Titration pH Value 5 - 7 pH Metry Solubility at Solvent Solubility ( gm/100ml ) Visual 25 °C Ethanol 56 Ethylene glycol 61 Isopropanol 41 Methanol 89 N-methylpyrrolidone 102 Propylene glycol methyl ether 61 Water 28 Product Applications: 1.POLYMER ADD-BRONOPOL is a broad-spectrum antimicrobial agent used as a preservative for topical cosmetics, pharmaceutical and toiletries. 2.It is a biocide widely used as a disinfectant, antibacterial, slime control and antiseptic ingredient for water treatment. 722/AB/15, Nirmal, Laxmi Park Colony, Navi Peth 411030, www.jpharmachem.com Tel. 020-24530071 Email:[email protected] Product Benifits: 1.POLYMER ADD-BRONOPOL has low toxicity profile. 2.It has extremely good efficacy against Pseudomonas, a weakness of isothiazolinone chemistry. -
Formaldehyde and Formaldehyde Releasers
INVESTIGATION REPORT FORMALDEHYDE AND FORMALDEHYDE RELEASERS SUBSTANCE NAME: Formaldehyde IUPAC NAME: Methanal EC NUMBER: 200-001-8 CAS NUMBER: 50-00-0 SUBSTANCE NAME(S): - IUPAC NAME(S): - EC NUMBER(S): - CAS NUMBER(S):- CONTACT DETAILS OF THE DOSSIER SUBMITTER: EUROPEAN CHEMICALS AGENCY Annankatu 18, P.O. Box 400, 00121 Helsinki, Finland tel: +358-9-686180 www.echa.europa DATE: 15 March 2017 CONTENTS INVESTIGATION REPORT – FORMALDEHYDE AND FORMALDEHYDE RELEASERS ................ 1 1. Summary.............................................................................................................. 1 2. Report .................................................................................................................. 2 2.1. Background ........................................................................................................ 2 3. Approach .............................................................................................................. 4 3.1. Task 1: Co-operation with FR and NL ..................................................................... 4 3.2. Task 2: Formaldehyde releasers ........................................................................... 4 4. Results of investigation ........................................................................................... 6 4.1. Formaldehyde..................................................................................................... 6 4.2. Information on formaldehyde releasing substances and their uses ............................ 6 4.2.1. -

(12) United States Patent (10) Patent No.: US 6,656,923 B1 Trinh Et Al
USOO6656923B1 (12) United States Patent (10) Patent No.: US 6,656,923 B1 Trinh et al. (45) Date of Patent: *Dec. 2, 2003 (54) UNCOMPLEXED CYCLODEXTRIN 5,464,545. A 11/1995 Isharani et al. .............. 252/8.6 COMPOSITIONS FOR ODOR AND WRINKLE CONTROL (List continued on next page.) FOREIGN PATENT DOCUMENTS (75) Inventors: Toan Trinh, Maineville, OH (US); Raymond Edward Bolich, Jr., DE 273.1520 1/1979 Maineville, OH (US); Helen Bernardo E. s s A: 5.08 Tordil, West Chester, OH (US); Robert EP O 354 648 B1 6/1994 Mermelstein, Cincinnati, OH (US); EP O 613 675 A1 9/1994 Marjorie Mossman Pefly, Cincinnati, FR 22O1880 5/1974 OH (US); Ricky Ah-Man Woo, JP SHO 53-41440 4/1978 Hamilton, OH (US); Daniel Scott JP SHO 58-1244.52 7/1983 Cobb, Loveland, OH (US); Eva JP SHO 61-128973 6/1986 Schneiderman, Fairfield, OH (US); JP SHO 63-164953 7/1988 Ann Margaret Wolff, Cincinnati, OH JP HEI 3-170415 7/1991 (US); Erin Lynn Rosenbalm, Fairfield, E. H 3. E. OH (US); Thomas Edward Ward, WO WO 96/04940 2/1996 Oxford, OH (US); Alex Haejoon WO WO95/17175 6/1996 Chung, West Chester, OH (US); Anthony James Burns, West Chester, OTHER PUBLICATIONS Qi's with Syril. Hashimoto, H., “Studies on the Industrial Production and Streutker Florence KY (US) Application of Cyclodextrins”, Starch Science, vol. 36, No. s s 1 (1989), pp. 35–42. Hashimoto, H., “Application of Cyclodextrins to Foods, (73) ASSignee: The Este, syll Company, Toiletries and Other Products in Japan', Ensuiko Sugar s Refining Co., Ltd., pp.