Incidence and Types of Bacteria Associated with Giblets of Commercially Processed Turkeys Richard Harry Salzer Iowa State University
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Avian Crop Function–A Review
Ann. Anim. Sci., Vol. 16, No. 3 (2016) 653–678 DOI: 10.1515/aoas-2016-0032 AVIAN CROP function – A REVIEW* * Bartosz Kierończyk1, Mateusz Rawski1, Jakub Długosz1, Sylwester Świątkiewicz2, Damian Józefiak1♦ 1Department of Animal Nutrition and Feed Management, Poznań University of Life Sciences, Wołyńska 33, 60-637 Poznań, Poland 2Department of Animal Nutrition and Feed Science, National Research Institute of Animal Production, 32-083 Balice n. Kraków, Poland ♦Corresponding author: [email protected] Abstract The aim of this review is to present and discuss the anatomy and physiology of crop in different avian species. The avian crop (ingluvies) present in most omnivorous and herbivorous bird spe- cies, plays a major role in feed storage and moistening, as well as functional barrier for pathogens through decreasing pH value by microbial fermentation. Moreover, recent data suggest that this gastrointestinal tract segment may play an important role in the regulation of the innate immune system of birds. In some avian species ingluvies secretes “crop milk” which provides high nutri- ents and energy content for nestlings growth. The crop has a crucial role in enhancing exogenous enzymes efficiency (for instance phytase and microbial amylase,β -glucanase), as well as the activ- ity of bacteriocins. Thus, ingluvies may have a significant impact on bird performance and health status during all stages of rearing. Efficient use of the crop in case of digesta retention time is es- sential for birds’ growth performance. Thus, a functionality of the crop is dependent on a number of factors, including age, dietary factors, infections as well as flock management. -

Ostrich Production Systems Part I: a Review
11111111111,- 1SSN 0254-6019 Ostrich production systems Food and Agriculture Organization of 111160mmi the United Natiorp str. ro ucti s ct1rns Part A review by Dr M.M. ,,hanawany International Consultant Part II Case studies by Dr John Dingle FAO Visiting Scientist Food and , Agriculture Organization of the ' United , Nations Ot,i1 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M-21 ISBN 92-5-104300-0 Reproduction of this publication for educational or other non-commercial purposes is authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without written permission of the copyright holders. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Information Division, Food and Agriculture Organization of the United Nations, Viale dells Terme di Caracalla, 00100 Rome, Italy. C) FAO 1999 Contents PART I - PRODUCTION SYSTEMS INTRODUCTION Chapter 1 ORIGIN AND EVOLUTION OF THE OSTRICH 5 Classification of the ostrich in the animal kingdom 5 Geographical distribution of ratites 8 Ostrich subspecies 10 The North -

A Disease Syndrome in Young Chickens 2-To 8-Weeks - Old Characterized by Erosion and Ulceration of the Gizzard Epithelial Linmg and Black Vomit Has Been Reported
Arch. Insh. Razi, 1981,32, 101-103 A CONDITION OF EROSION AND ULCERATION OF YOUNG CHICKEN'S GIZZARD IN IRAN By: M. Farshian SUMMARY: A disease syndrome in young chickens 2-to 8-weeks - old characterized by erosion and ulceration of the gizzard epithelial linmg and black vomit has been reported. The presence of a dark brown - co!oured fluid in the crop, proventri cul us, gizzard and small intestine was oftenly observed. Tue syndrome caused considerable mortaility losses and reduced weight gain in broilers. INTRODUCTION A few reports from the U.S.A. and Latin Amtrican countries have described a disease syndrome in young chickens known to poultrymen in latter terri tories as « Vomito Negro» or black vomit ( Cover and Paredes, 1971; Johnson and Pinedo; 1971). In Iran a condition very similar to the above mentioned syndrome, coming into being occasionally noticed in the past year or 50, has increased in incidence during the past six months, beginning September 1980. The following is an account of the clinical and gross pathological findings of the syndrome. Clinical signs : Affected chickens, 2-to 8 - weeks - old appeared depressed, lost their appetite and usually had pale combs and wattles. Birds were frequently Wlable to stand and sorne had their necks stretched on the groWld. A dark - coloured diarrhea was not uncommon. Death usually occurred within few hours from the onset of the symptoms. 101 The morbidity rate vearried from 5 % to 25 % and daily mortality ranged from 0.1 % to 1% The disease took a 2-to 3 - week course after which time it appeared that the birds developed sorne sort of resistance to the condition. -

An Important Health and Welfare Issue of Growing Ostriches
DOI: 10.2478/ats-2020-0016 AGRICULTURA TROPICA ET SUBTROPICA, 53/4, 161–173, 2020 Review Article Gastric impaction: an important health and welfare issue of growing ostriches Muhammad Irfan1, Nasir Mukhtar2, Tanveer Ahmad3, Muhammad Tanveer Munir4 1College of Veterinary Medicine, Kyungpook National University, Daegu 41566, Republic of Korea 2Department of Poultry Sciences (Station for Ostrich research & Development), Faculty of Veterinary and Animal Sciences, PMAS‑Arid Agriculture University, Rawalpindi, Pakistan 3Department of Clinical Sciences, Faculty of Veterinary Sciences, Bahauddin Zakariya University, Multan, Pakistan 4LIMBHA, Ecole Supérieur du Bois, 44306 Nantes, France Correspondence to: M. T. Munir, LIMBHA, Ecole Supérieur du Bois, 7 Rue Christian Pauc, 44306 Nantes, France. E‑mail: [email protected] Abstract Ostrich farming serves as a source for meat, feathers, skin, eggs, and oil. In general, ostriches are hardy birds that can resist a wide range of climatic harshness and some diseases. However, musculoskeletal and digestive complications, including the gastric impaction, remain the major cause of mortality. The gastrointestinal impaction alone is responsible for 30 – 46% of spontaneous deaths in growing ostriches. The literature review of 21 publications on this subject has shown that 90% of these incidents happen during first six months of life. The aetiology of this problem is mostly stress and behaviour‑related gorging of feed and picking on non‑feeding materials such as stone, sand, wood pieces, plastic, glass, and metallic objects. Conservative therapy or surgical approaches show good results with almost 70 to 100% recovery depending upon the clinical presentation and timely diagnosis. Overall, this literature review describes impaction in farmed ostriches, along with diagnosis, treatment, and control and preventive measures. -

A Simulated Bird Gastric Mill and Its Implications for Fossil Gastrolith Authenticity
Fossil Record 12 (1) 2009, 91–97 / DOI 10.1002/mmng.200800013 A simulated bird gastric mill and its implications for fossil gastrolith authenticity Oliver Wings Steinmann-Institut fr Geologie, Mineralogie und Palontologie, Universitt Bonn, Nussallee 8, 53115 Bonn, Germany. E-mail: [email protected] Current address: Museum fr Naturkunde Berlin, Invalidenstraße 43, 10115 Berlin, Germany. Abstract Received 12 June 2008 A rock tumbler, stones, water, plant material, hydrochloric acid, and pepsin were used Accepted 15 August 2008 to simulate a bird gizzard in order to study abrasion rate and influence of stomach Published 20 February 2009 juices and foodstuff on gastrolith surface development. The experiment lasted for six months. Each week, the “stomach” was supplied with fresh grass and stomach juices. After the end of the experiment, the set of stones had a combined weight loss of 22.4%, with softer rock types showing higher abrasion rates. The combination of sto- mach juices and silica phytoliths within the grass had no visible effect on stone surface development: polish or pitting did not occur. A second experiment combined only peb- bles with water in the tumbler. Results indicate that rock abrasion is mainly caused by contacts between moving stones. A comparison with authentic ostrich gastroliths Key Words showed that abrasion in the artificial stomach must have been lower than in a real gizzard, but still too high to maintain or develop surface polish. If high polish occa- stomach stones sionally seen on sauropodomorph dinosaur gastroliths was indeed caused in a stomach artificial gizzard environment, it implies digestive processes different from those of extant birds and the clast surface development “artificial gizzard”. -

Relationships Between Carcass Traits and Offal Components in Local Poultry Populations of Benin Journal of Applied Biosciences 69:5 510 – 5522
Tougan et al . J. Appl. Biosci. 2013 . Relationships between carcass traits and offal components in local poultry populations of Benin Journal of Applied Biosciences 69:5 510 – 5522 ISSN 1997–5902 Relationships between carcass traits and offal components in local poultry populations (gallus gallus ) of Benin Tougan P U 1, Dahouda M 2, Salifou C F A 1, Ahounou G S 1, Kpodekon M T 1, Mensah G A 3, Kossou D N F 1, Amenou C 1, Kogbeto C E 1, Lognay G 4, Thewis 5, Youssao I A K 1 1 Department of Animal Production and Health, Polytechnic School of Abomey-Calavi, 01 BP 2009,Cotonou, Republic of Benin. ² Department of Animal Production, Faculty of Agronomic Science, University of Abomey-Calavi, 01 BP 526, Republic of Benin. 3 Agricultural Research Center of Agonkanmey, National Institute of Agricultural Research of Benin, 01 BP 884, Cotonou 01, Republic of Benin. 4 Laboratory of Analytical Chemistry, Gembloux Agro-Bio Tech, University of Liège, 2, Passage des Déportés -5030 Gembloux, Belgique. 5 Animal Sciences Unit, Gembloux Agro-Bio Tech, University of Liege, Passage des Déportés, 2, 5030 Gembloux, Belgium.. * Correspondence Prof. Issaka YOUSSAO ABDOU KARIM / EPAC / Department of Animal Production and Health, Polytechnic School of Abomey-Calavi, 01 BP 2009,Cotonou, Republic of Benin. Phone : 00 229 95 28 59 88 ou 00 229 97 91 20 74, Fax : 00 229 21 36 01 99. E-mail : [email protected] , [email protected] Original submitted in on 13 th August 2013 Published online at www.m.elewa.org on 30 th September 2013. -

Accumulation Patterns and Risk Assessment of Metals and Metalloid in T Muscle and Offal of Free-Range Chickens, Cattle and Goat in Benin City, Nigeria
Ecotoxicology and Environmental Safety 151 (2018) 98–108 Contents lists available at ScienceDirect Ecotoxicology and Environmental Safety journal homepage: www.elsevier.com/locate/ecoenv Accumulation patterns and risk assessment of metals and metalloid in T muscle and offal of free-range chickens, cattle and goat in Benin City, Nigeria Emmanuel Temiotan Ogbomidaa, Shouta M.M. Nakayamab, Nesta Bortey-Samb, Balazs Oroszlanyb, Isioma Tongoc, Alex Ajeh Enunekuc, Ogbeide Ozekekec, Martins Oshioriamhe Aineruac, Iriagbonse Priscillia Fasipea, Lawrence Ikechukwu Ezemonyec, ⁎ Hazuki Mizukawab, Yoshinori Ikenakab,d, Mayumi Ishizukab, a Ecotoxicology and Environmental Forensic Unit, National Centre for Energy and Environment, Energy Commission of Nigeria, University of Benin, P.M.B 1154, Benin City, Nigeria b Laboratory of Toxicology, Department of Environmental Veterinary Sciences, Graduate School of Veterinary Medicine, Hokkaido University, Kita 18, Nishi 9, Kita ku, Sapporo 0600818, Japan c Department of Animal and Environmental Biology (AEB), University of Benin, P.M.B 1154, Benin City, Nigeria d Water Research Group, Unit for Environmental Sciences and Management, North-West University, Potchefstroom, South Africa ARTICLE INFO ABSTRACT Keywords: The use of free range animals for monitoring environmental health offers opportunities to detect exposure and Heavy metals assess the toxicological effects of pollutants in terrestrial ecosystems. Potential human health risk of dietary Offal intake of metals and metalloid via consumption of offal and muscle of free range chicken, cattle and goats by the Muscles urban population in Benin City was evaluated. Muscle, gizzard, liver and kidney samples were analyzed for Cr, Hazard Quotient Mn, Fe, Co, Ni, Cu, Zn, As, Cd, and Pb concentrations using inductively coupled plasma mass spectrometer (ICP- Hazard Index MS) while Hg was determined using Hg analyzer. -

The Gizzard (Kirkiban)
The Gizzard (kirkiban) The kirkiban or gizzard, is the stomach of the bird. Most birds eat primarily seeds which need to be digested. As a prerequisite to digestion the seeds must be broken down. For this reason, many birds swallow pebbles, which are rubbed against the seeds which the bird eats. The gizzard is a very muscular organ, as it must be able Gizzard 1:1 Gizzard 1:2 Gizzard 1:3 to grind the seeds to s digestible form. Inspecting the Gizzard If the gizzard is punctured, then it is forbidden. It is not unusually for birds to swallow nails or other objects which can cause a rupture in the gizzard. Particularly, ducks and geese, tend to swallow large metal objects and as such their gizzards should be inspected. If there is a problem in the gizzard, there is usually a significant amount of inflammation, and thus it is quickly noticed. In the event that there is such an inflammation, and there is the possibility that something punctured the gizzard a rabbinical authority should be consulted. Kashering the Gizzard The gizzard is essentially a muscle, the inside is lined by a latex like substance. This inside membrane is very rough and not edible. To kosher the gizzard, cut the gizzard open, remove the inside membrane and then soak and salt the gizzard as you Gizzard 1:4 would any other piece of meat. How the Gizzard is used to Identify Kosher Birds One of the physical characteristics of kosher birds is that they have a gizzard which is composed of the two membranes just described. -

Stomach Histology of Crocodylus Siamensisand Gavialis Gangeticus Reveals Analogy of Archosaur
Acta Herpetologica 15(2): 111-118, 2020 DOI: 10.13128/a_h-7564 Stomach histology of Crocodylus siamensis and Gavialis gangeticus reveals analogy of archosaur “gizzards”, with implication on crocodylian gastroliths function Ryuji Takasaki1,2,*, Yoshitsugu Kobayashi3 1 Department of Natural History and Planetary Sciences, Hokkaido University, Kita 10, Nishi 8, Kita-ku, Sapporo, Hokkaido, 060-0810, Japan 2 Faculty of Biosphere-Geosphere Science, Okayama University of Science, Ridai-cho, Kita-ku, Okayama, Japan 3 Hokkaido University Museum, Hokkaido University, Kita 10, Nishi 8, Kita-ku, Sapporo, Hokkaido, 060-0810, Japan *Corresponding author. E-mail: [email protected] Submitted on: 2020, 1st December; revised on: 2020, 14th August; accepted on: 2020, 1st October Editor: Emilio Sperone Abstract. Two groups of extant Archosauria, Crocodylia and Neornithes, have two-chambered stomachs and store gastroliths inside their “gizzards”. Morphological similarities of the “gizzards” lead some previous studies to assume that the presence of this structure, organ “gizzard” is synapomorphic to Archosauria. However, the homology of archosaur “gizzards” had never been tested. This study provides general histological descriptions of stomachs of two crocodylian taxa, Crocodylus siamensis and Gavialis gangeticus, to determine the homology of crocodylian and neor- nithine “gizzards”. Our study demonstrates that both Crocodylus siamensis and Gavialis gangeticus have longer, more complex glands in the fundic stomach (crocodylian “gizzard”) than in the pyloric stomach. Additionally, we found that compound glands are present in the fundic stomach of Crocodylus siamensis. Therefore, crocodylian stomach histomorphological structures are concordant with those of other non-avian reptiles, despite the unique gross mor- phology. The pyloric regions of non-avian reptile stomachs are known to be homologous with the pyloric regions of mammalian stomachs as well as neornithine ventriculus (neornithine gizzard). -

Our Local Lunch Starters
LIGHT BITES/APPETISERS OUR LOCAL LUNCH Original Chef’s Special (S) GHS 45.00 (grilled marinated chicken pieces on the bone tossed in spicy (EFIE NI EFIE) chef’s special sauce) Chicken Gizzard GHS 40.00 SOUPS (Nkwan) (soft/crispy chicken gizzards tossed in delicious local spices) (All served with a choice of side order) Grilled Chicken Wings GHS 45.00 (BBQ/ Spicy) Aponkyi Nkakra GHS 50.00 Akonfem - Half GHS 55.00 (goat meat & intestines cooked in delicious tomato light soup) (Grilled – wine marinated guinea fowl halves tossed with local Nkatse GHS 50.00 spices) (Groundnut soup with a choice of fish, meat or chicken) Akonfem - Full GHS 95.00 Abe GHS 50.00 (Grilled – wine marinated full guinea fowl tossed with local (Palmnut soup with a choice of fish, meat or chicken) spices) Tilapia GHS 55.00 Lamb Donner Kebab (S) GHS 65.00 (Light soup with fresh tilapia fish) (Imported lamb donner kebab on the grill) PALAVA SAUCE GHS 50.00 Yam Balls GHS 25.00 FANTE FANTE GHS 65.00 (deep fried mashed yam with vegetables coated in bread crumbs) RED RED GHS 40.00 Kelewele GHS 25.00 (deep fried ripe plantain dices tossed in ginger & local spices) EXTRAS SUYA (KEBABS) Suya – Fish (S) GHS 50.00 Snails GHS 25.00 (Well marinated chargrilled fish fillet chunks on a skewer) Tilapia GHS 20.00 Suya - Beef GHS 45.00 Dry Fish GHS 20.00 (char-roast beef fillet slices with local spicy seasoning in a wrap) Mushroom GHS 15.00 Suya – Chicken GHS 45.00 Aponkyi GHS 12.00 (Char-grilled chicken breast chunks seasoned with local spices on a skewer) NB: 15% Entertainment Charge applies on total bill during performance /event nights. -

Comparison of Qualitative and Quantitative Properties of the Wings, Necks and Offal of Chicken Broilers from Organic and Conventional Production Systems
Veterinarni Medicina, 61, 2016 (11): 643–651 Original Paper doi: 10.17221/286/2015-VETMED Comparison of qualitative and quantitative properties of the wings, necks and offal of chicken broilers from organic and conventional production systems F.A.A. Abdullah, H. Buchtova University of Veterinary and Pharmaceutical Sciences Brno, Brno, Czech Republic ABSTRACT: The aim of the present study was to investigate qualitative and quantitative properties of wings, offal (liver, heart and gizzard) and necks of chickens from organic and conventional production systems, currently avail- able on the market for Czech consumers. Production properties (yield and weight), surface colour (lightness, red- ness, yellowness) and chemical indicators (dry matter, total protein, net protein, collagen, hydroxyproline, fat, ash and phosphorus) were evaluated in fresh chicken broilers. Conventionally produced chickens had higher carcass yields but higher wing yields and weights were observed in organic broilers. The skin, bones, tip (left wings) and the meat with skin (right wings) of organic broilers were heavier (P < 0.05) than those of conventional chickens. The dry matter and total protein content of deboned organic broiler wings (meat with skin) was greater (P < 0.01) than those of conventional wings. Similarly as for yields, the offal (heart, gizzard) and necks of organic chickens had significantly (P < 0.01) higher weights in comparison with conventional chickens. Colour indicators showed that the external surface of the livers, necks and gizzards (muscle) from organic chickens were darker (lightness; P < 0.01). Total protein content in livers, hearts and necks of organic chickens was greater; fat content in the livers and necks of organic broilers was also higher (P < 0.05) than those of conventional broilers. -
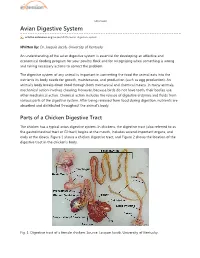
Avian Digestive System
eXtension Avian Digestive System articles.extension.org/pages/65376/avian-digestive-system Written by: Dr. Jacquie Jacob, University of Kentucky An understanding of the avian digestive system is essential for developing an effective and economical feeding program for your poultry flock and for recognizing when something is wrong and taking necessary actions to correct the problem. The digestive system of any animal is important in converting the food the animal eats into the nutrients its body needs for growth, maintenance, and production (such as egg production). An animal's body breaks down food through both mechanical and chemical means. In many animals, mechanical action involves chewing; however, because birds do not have teeth, their bodies use other mechanical action. Chemical action includes the release of digestive enzymes and fluids from various parts of the digestive system. After being released from food during digestion, nutrients are absorbed and distributed throughout the animal's body. Parts of a Chicken Digestive Tract The chicken has a typical avian digestive system. In chickens, the digestive tract (also referred to as the gastrointestinal tract or GI tract) begins at the mouth, includes several important organs, and ends at the cloaca. Figure 1 shows a chicken digestive tract, and Figure 2 shows the location of the digestive tract in the chicken's body. Fig. 1. Digestive tract of a female chicken. Source: Jacquie Jacob, University of Kentucky. Fig. 2. Location of the digestive tract in a female chicken. Source: Public domain. Beak/Mouth As with most birds, a chicken obtains feed by using its beak. Food picked up by the beak enters the mouth.