Opposing Roles for JNK and Aurora a in Regulating the Association Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Identification and Characterization of Genes Essential for Human Brain Development
Identification and Characterization of Genes Essential for Human Brain Development The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Ganesh, Vijay S. 2012. Identification and Characterization of Genes Essential for Human Brain Development. Doctoral dissertation, Harvard University. Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:9773743 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA Copyright © 2012 by Vijay S. Ganesh All rights reserved. Dissertation Advisor: Dr. Christopher A. Walsh Author: Vijay S. Ganesh Identification and Characterization of Genes Essential for Human Brain Development Abstract The human brain is a network of ninety billion neurons that allows for many of the behavioral adaptations considered unique to our species. One-fifth of these neurons are layered in an epithelial sheet known as the cerebral cortex, which is exquisitely folded into convolutions called gyri. Defects in neuronal number clinically present with microcephaly (Greek for “small head”), and in inherited cases these defects can be linked to mutations that identify genes essential for neural progenitor proliferation. Most microcephaly genes are characterized to play a role in the centrosome, however rarer presentations of microcephaly have identified different mechanisms. Charged multivesicular body protein/Chromatin modifying protein 1A (CHMP1A) is a member of the ESCRT-III endosomal sorting complex, but is also suggested to localize to the nuclear matrix and regulate chromatin. -

Molecular Genetics of Microcephaly Primary Hereditary: an Overview
brain sciences Review Molecular Genetics of Microcephaly Primary Hereditary: An Overview Nikistratos Siskos † , Electra Stylianopoulou †, Georgios Skavdis and Maria E. Grigoriou * Department of Molecular Biology & Genetics, Democritus University of Thrace, 68100 Alexandroupolis, Greece; [email protected] (N.S.); [email protected] (E.S.); [email protected] (G.S.) * Correspondence: [email protected] † Equal contribution. Abstract: MicroCephaly Primary Hereditary (MCPH) is a rare congenital neurodevelopmental disorder characterized by a significant reduction of the occipitofrontal head circumference and mild to moderate mental disability. Patients have small brains, though with overall normal architecture; therefore, studying MCPH can reveal not only the pathological mechanisms leading to this condition, but also the mechanisms operating during normal development. MCPH is genetically heterogeneous, with 27 genes listed so far in the Online Mendelian Inheritance in Man (OMIM) database. In this review, we discuss the role of MCPH proteins and delineate the molecular mechanisms and common pathways in which they participate. Keywords: microcephaly; MCPH; MCPH1–MCPH27; molecular genetics; cell cycle 1. Introduction Citation: Siskos, N.; Stylianopoulou, Microcephaly, from the Greek word µικρoκεϕαλi´α (mikrokephalia), meaning small E.; Skavdis, G.; Grigoriou, M.E. head, is a term used to describe a cranium with reduction of the occipitofrontal head circum- Molecular Genetics of Microcephaly ference equal, or more that teo standard deviations -

Ncomms4885.Pdf
ARTICLE Received 4 Feb 2014 | Accepted 14 Apr 2014 | Published 30 May 2014 DOI: 10.1038/ncomms4885 Microcephaly disease gene Wdr62 regulates mitotic progression of embryonic neural stem cells and brain size Jian-Fu Chen1,2, Ying Zhang1, Jonathan Wilde1, Kirk C. Hansen3, Fan Lai4 & Lee Niswander1 Human genetic studies have established a link between a class of centrosome proteins and microcephaly. Current studies of microcephaly focus on defective centrosome/spindle orientation. Mutations in WDR62 are associated with microcephaly and other cortical abnormalities in humans. Here we create a mouse model of Wdr62 deficiency and find that the mice exhibit reduced brain size due to decreased neural progenitor cells (NPCs). Wdr62 depleted cells show spindle instability, spindle assembly checkpoint (SAC) activation, mitotic arrest and cell death. Mechanistically, Wdr62 associates and genetically interacts with Aurora A to regulate spindle formation, mitotic progression and brain size. Our results suggest that Wdr62 interacts with Aurora A to control mitotic progression, and loss of these interactions leads to mitotic delay and cell death of NPCs, which could be a potential cause of human microcephaly. 1 Howard Hughes Medical Institute, Department of Pediatrics, University of Colorado Anschutz Medical Campus, Children’s Hospital Colorado, Aurora, Colorado 80045, USA. 2 Department of Genetics, Department of Biochemistry & Molecular Biology, University of Georgia, Athens, Georgia 30602, USA. 3 Biochemistry & Molecular Genetics, University of Colorado Denver, Aurora, Colorado 80045, USA. 4 The Wistar Institute, 3601 Spruce Street, Philadelphia, Pennsylvania 19104, USA. Correspondence and requests for materials should be addressed to J-F.C. (email: [email protected]) or to L.N. -

(JNK)-Binding Protein WDR62 Is Recruited to Stress Granules And
Molecular Biology of the Cell Vol. 21, 117–130, January 1, 2010 A Novel c-Jun N-terminal Kinase (JNK)-binding Protein WDR62 Is Recruited to Stress Granules and Mediates a Nonclassical JNK Activation Tanya Wasserman,*† Ksenya Katsenelson,*† Sharon Daniliuc,*† Tal Hasin,* Mordechay Choder,‡ and Ami Aronheim* Departments of *Molecular Genetics and ‡Microbiology, The Rappaport Family Institute for Research in the Medical Sciences, Technion-Israel Institute of Technology, Haifa 31096, Israel Submitted June 22, 2009; Revised October 27, 2009; Accepted October 29, 2009 Monitoring Editor: Jonathan Chernoff The c-Jun N-terminal kinase (JNK) is part of a mitogen-activated protein kinase (MAPK) signaling cascade. Scaffold proteins simultaneously associate with various components of the MAPK signaling pathway and play a role in signal transmission and regulation. Here we describe the identification of a novel scaffold JNK-binding protein, WDR62, with no sequence homology to any of the known scaffold proteins. WDR62 is a ubiquitously expressed heat-sensitive 175-kDa protein that specifically associates with JNK but not with ERK and p38. Association between WDR62 and JNKs occurs in the absence and after either transient or persistent stimuli. WDR62 potentiates JNK kinase activity; however it inhibits AP-1 transcription through recruitment of JNK to a nonnuclear compartment. HEK-293T cells transfected with WDR62 display cytoplasmic granular localization. Overexpression of stress granule (SG) resident proteins results in the recruit- ment of endogenous WDR62 and activated JNK to SG. In addition, cell treatment with arsenite results in recruitment of WDR62 to SG and activated JNK to processing bodies (PB). JNK inhibition results in reduced number and size of SG and reduced size of PB. -

Cenpj Regulates Cilia Disassembly and Neurogenesis in the Developing Mouse Cortex
This Accepted Manuscript has not been copyedited and formatted. The final version may differ from this version. A link to any extended data will be provided when the final version is posted online. Research Articles: Development/Plasticity/Repair Cenpj regulates cilia disassembly and neurogenesis in the developing mouse cortex Wenyu Ding1,2, Qian Wu1,2, Le Sun1,2, Na Clara Pan1,2 and Xiaoqun Wang1,2,2 1State Key Laboratory of Brain and Cognitive Science, CAS Center for Excellence in Brain Science and Intelligence Technology (Shanghai), Institute of Biophysics, Chinese Academy of Sciences, Beijing, 100101, China 2University of Chinese Academy of Sciences, Beijing 100049, China 3Beijing Institute for Brain Disorders, Beijing 100069, China https://doi.org/10.1523/JNEUROSCI.1849-18.2018 Received: 20 July 2018 Revised: 19 December 2018 Accepted: 24 December 2018 Published: 9 January 2019 Author contributions: W.D., Q.W., and X.W. designed research; W.D., Q.W., L.S., N.C.P., and X.W. performed research; W.D., Q.W., L.S., N.C.P., and X.W. analyzed data; W.D., Q.W., L.S., N.C.P., and X.W. edited the paper; W.D., Q.W., and X.W. wrote the paper; Q.W. and X.W. wrote the first draft of the paper; X.W. contributed unpublished reagents/analytic tools. Conflict of Interest: The authors declare no competing financial interests. We gratefully acknowledge Dr. Bradley Yoder (University of Alabama at Birmingham) kindly shared and transferred the mouse strain to us. We thank Dr. Tian Xue (University of Science and Technology of China) for sharing ARPE19 cell line with us. -

A Novel Single Base Pair Duplication in WDR62 Causes Primary Microcephaly Verena Rupp1, Sobiah Rauf2, Ishrat Naveed2, Windpassinger Christian1 and Asif Mir2*
Rupp et al. BMC Medical Genetics 2014, 15:107 http://www.biomedcentral.com/1471-2350/15/107 CASE REPORT Open Access A novel single base pair duplication in WDR62 causes primary microcephaly Verena Rupp1, Sobiah Rauf2, Ishrat Naveed2, Windpassinger Christian1 and Asif Mir2* Abstract Background: Primary microcephaly is a disorder of the brain resulting in a reduced head circumference that can come along with intellectual disability but with hardly any other neurological abnormalities. Case presentation: In this study we report on three Pakistani males from a consanguineous family with 2, 4 and 25 years, diagnosed with autosomal recessive primary microcephaly. By genotyping, Sanger sequencing and using bioinformatical approaches the disease causing mutation was identified and evaluated. Conclusion: By using a 250K SNP array, we were able to detect an 11Mb large autozygous region in the MCPH2 locus on chromosome 19q13.12. Sequencing of the associated gene, WDR62, revealed the frameshift causing single base pair duplication, c.2527dupG. This mutation is predicted to affect the structural features of WDR62 which in turn changes the conformation and function of the protein. Aspartic acid (D) at position 843 was found to be conserved among various ortholog species. The present findings will be helpful in genetic diagnosis of patients and future studies of WDR62. Keywords: Autosomal recessive primary microcephaly (MCPH), MCPH2 locus, WDR62, Mutation Background Although sloping foreheads and reduced intelligence are Autosomal recessive primary microcephaly (MCPH) is a very common, they are not listed as a basic criteria for the rare malformation of the head resulting in a circumfer- diagnosis of microcephaly [19,22]. -

Modifier Genes in Microcephaly: a Report on WDR62, CEP63, RAD50
G C A T T A C G G C A T genes Article Modifier Genes in Microcephaly: A Report on WDR62, CEP63, RAD50 and PCNT Variants Exacerbating Disease Caused by Biallelic Mutations of ASPM and CENPJ Ehtisham Ul Haq Makhdoom 1,2,3,†, Syeda Seema Waseem 1,4,†, Maria Iqbal 1,2,4, Uzma Abdullah 5 , Ghulam Hussain 3, Maria Asif 1,4, Birgit Budde 1 , Wolfgang Höhne 1, Sigrid Tinschert 6, Saadia Maryam Saadi 2 , Hammad Yousaf 2, Zafar Ali 7, Ambrin Fatima 8, Emrah Kaygusuz 9 , Ayaz Khan 2 , Muhammad Jameel 2, Sheraz Khan 2 , Muhammad Tariq 2 , Iram Anjum 10 , Janine Altmüller 1, Holger Thiele 1, Stefan Höning 4, Shahid Mahmood Baig 2,8,11, Peter Nürnberg 1,12 and Muhammad Sajid Hussain 1,4,12,* 1 Cologne Center for Genomics (CCG), Faculty of Medicine and University Hospital Cologne, University of Cologne, 50931 Cologne, Germany; [email protected] (E.U.H.M.); [email protected] (S.S.W.); [email protected] (M.I.); [email protected] (M.A.); [email protected] (B.B.); [email protected] (W.H.); [email protected] (J.A.); [email protected] (H.T.); [email protected] (P.N.) 2 Human Molecular Genetics Laboratory, Health Biotechnology Division, National Institute for Biotechnology and Genetic Engineering (NIBGE) College, PIEAS, Faisalabad 38000, Pakistan; [email protected] (S.M.S.); [email protected] (H.Y.); [email protected] (A.K.); [email protected] (M.J.); [email protected] (S.K.); [email protected] (M.T.); [email protected] (S.M.B.) 3 Citation: Makhdoom, E.U.H.; Neurochemicalbiology and Genetics Laboratory (NGL), Department of Physiology, Faculty of Life Sciences, Waseem, S.S.; Iqbal, M.; Abdullah, U.; Government College University, Faisalabad 38000, Pakistan; [email protected] 4 Hussain, G.; Asif, M.; Budde, B.; Institute of Biochemistry I, Medical Faculty, University of Cologne, 50931 Cologne, Germany; [email protected] Höhne, W.; Tinschert, S.; Saadi, 5 University Institute of Biochemistry and Biotechnology (UIBB), PMAS-Arid Agriculture University, S.M.; et al. -

UC San Francisco Previously Published Works
UCSF UC San Francisco Previously Published Works Title Whole-exome sequencing identifies recessive WDR62 mutations in severe brain malformations. Permalink https://escholarship.org/uc/item/8tg8p58p Journal Nature, 467(7312) ISSN 0028-0836 Authors Bilgüvar, Kaya Oztürk, Ali Kemal Louvi, Angeliki et al. Publication Date 2010-09-01 DOI 10.1038/nature09327 Peer reviewed eScholarship.org Powered by the California Digital Library University of California HHS Public Access Author manuscript Author Manuscript Author ManuscriptNature. Author ManuscriptAuthor manuscript; Author Manuscript available in PMC 2011 July 04. Published in final edited form as: Nature. 2010 September 9; 467(7312): 207–210. doi:10.1038/nature09327. Whole exome sequencing identifies recessive WDR62 mutations in severe brain malformations Kaya Bilgüvar1,2,3,#, Ali Kemal Öztürk1,2,3,#, Angeliki Louvi1,2, Kenneth Y Kwan2,4, Murim Choi3, Burak Tatli5, Dilek Yalnizoğlu6, Beyhan Tüysüz7, Ahmet Okay Çağlayan8, Sarenur Gökben9, Hande Kaymakçalan10, Tanyeri Barak1,2,3, Mehmet Bakircioğlu1,2,3, Katsuhito Yasuno1,2,3, Winson Ho1,2,3, Stephan Sanders3,11,12, Ying Zhu2,4, Sanem Yilmaz9, Alp Dinçer13, Michele H Johnson1,14,15, Richard A Bronen1,14, Naci Koçer16, Hüseyin Per17, Shrikant Mane18, Mehmet Necmettin Pamir19, Cengiz Yalçinkaya20, Sefer Kumandaş17, Meral Topçu6, Meral Özmen5, Nenad Šestan2,4, Richard P Lifton3,21,*, Matthew W State3,11,12,*, and Murat Günel1,2,3,* 1Department of Neurosurgery, Program on Neurogenetics, Yale University School of Medicine, New Haven CT, 06510, USA -

Whole-Exome Sequencing Identifies Recessive WDR62 Mutations in Severe Brain Malformations
Vol 467 | 9 September 2010 | doi:10.1038/nature09327 LETTERS Whole-exome sequencing identifies recessive WDR62 mutations in severe brain malformations Kaya Bilgu¨var1,2,3*, Ali Kemal O¨ ztu¨rk1,2,3*, Angeliki Louvi1,2,3, Kenneth Y. Kwan2,4, Murim Choi3, Burak Tatlı5, Dilek Yalnızog˘lu6, Beyhan Tu¨ysu¨z7, Ahmet Okay C¸ag˘layan8, Sarenur Go¨kben9, Hande Kaymakc¸alan10, Tanyeri Barak1,2,3, Mehmet Bakırcıog˘lu1,2,3, Katsuhito Yasuno1,2,3, Winson Ho1,2,3, Stephan Sanders3,11,12, Ying Zhu2,4, Sanem Yılmaz9, Alp Dinc¸er13, Michele H. Johnson1,14,15, Richard A. Bronen1,14, Naci Koc¸er16, Hu¨seyin Per17, Shrikant Mane3,18, Mehmet Necmettin Pamir19, Cengiz Yalc¸ınkaya20, Sefer Kumandas¸17, Meral Topc¸u6, Meral O¨ zmen5, Nenad Sˇestan2,4, Richard P. Lifton3,21, Matthew W. State3,11,12 & Murat Gu¨nel1,2,3 The development of the human cerebral cortex is an orchestrated proliferation, migration or organization. Application of traditional process involving the generation of neural progenitors in the mapping approaches has proved to be particularly challenging for gene periventricular germinal zones, cell proliferation characterized discovery in these syndromes, where kindreds with a single affected by symmetric and asymmetric mitoses, followed by migration of member are most common, linkage studies support high locus hetero- post-mitotic neurons to their final destinations in six highly geneity and recent genetic findings have fundamentally challenged ordered, functionally specialized layers1,2. An understanding of previous diagnostic nosology3,7,8. Based on the expectation that whole- the molecular mechanisms guiding these intricate processes is in exome sequencing using next generation platforms9–11 can markedly its infancy, substantially driven by the discovery of rare mutations improve gene discovery efforts in these situations, we applied this that cause malformations of cortical development3–6. -
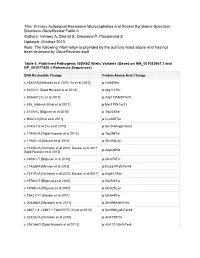
Title: Primary Autosomal Recessive Microcephalies and Seckel
Title: Primary Autosomal Recessive Microcephalies and Seckel Syndrome Spectrum Disorders GeneReview Table 6 Authors: Verloes A, Drunat S, Gressens P, Passemard S Updated: October 2013 Note: The following information is provided by the authors listed above and has not been reviewed by GeneReviews staff. Table 6. Published Pathogenic WDR62 Allelic Variants (Based on NM_001083961.1 and NP_001077430.1 Reference Sequences) DNA Nucleotide Change Protein Amino Acid Change c.193G>A [Nicholas et al 2010, Yu et al 2010] p.Val65Met c.332G>C [Sajid Hussain et al 2013] p.Arg111Thr c.363delT [Yu et al 2010] p.Asp112MetfsTer5 c.535_536insA [Bhat et al 2011] p.Met179fsTer21 c.671G>C [Bilguvar et al 2010] p.Trp224Ser c.900C>A [Bhat et al 2011] p.Cys300Ter c.1043+1G>A [Yu et al 2010] p.Ser348ArgfsTer63 c.1194G>A [Sajid Hussain et al 2013] p.Trp398Ter c.1198G >A [Bacino et al 2012] p.Gln400Lys c.1313G>A [Nicholas et al 2010, Kousar et al 2011, p.Arg438His Sajid Hussain et al 2013] c.1408C>T [Bilguvar et al 2010] p.Gln470Ter c.1143delA [Memon et al 2013] p.His381ProfsTer48 c.1531G>A [Nicholas et al 2010, Kousar et al 2011] p.Asp511Asn c.1576G>T [Bilguvar et al 2010] p.Glu526Ter c.1576G>A [Bilguvar et al 2010] p.Glu526Lys c.1942 C>T [Kousar et al 2011] p.Gln648Ter c.2083delA [Murdock et al 2011] p.Ser696AlafsTer4 c.2867 + 4_c2867 + 7delGGTG [Yu et al 2010] p.Ser956CysfsTer38 c.3232G>A [Nicholas et al 2010] p.Ala1078Thr c.3361delG [Sajid Hussain et al 2013] p.Ala1121GlnfsTer6 DNA Nucleotide Change Protein Amino Acid Change c.3503G>A [Sajid Hussain et al 2013] p.Trp1168 c.3839_3855delGCCAAGAGCCTGCCCTG YU p.Gly1280AfsTer21 [Bilguvar et al 2010] c.3936dupC/c.3936_3937insC [Yu et al 2010, p.Val1314ArgfsTer18/p.Val1313GlyfsTer17 Kousar et al 2011] c.4205delTGCC [Bilguvar et al 2010, Nicholas et al p.Val1402GlyfsTer12 2010] c.4241dupT [Bilguvar et al 2010] p.Leu1414LeufsTer41 See Quick Reference for an explanation of nomenclature. -

Aurora a Protein Kinase: to the Centrosome and Beyond
Review Aurora A Protein Kinase: To the Centrosome and Beyond Laura Magnaghi-Jaulin, Grégory Eot-Houllier, Emmanuel Gallaud and Régis Giet * University of Rennes, CNRS UMR 6290, IGDR-Institute of Genetics and Development of Rennes, F-35000 Rennes, France; [email protected] (L.M.-J.); [email protected] (G.E.-H.); [email protected] (E.G.) * Correspondence: [email protected] (R.G.); Tel +33223234998 Received: 7 December 2018; Accepted: 9 January 2019; Published: 15 January 2019 Abstract: Accurate chromosome segregation requires the perfect spatiotemporal rearrangement of the cellular cytoskeleton. Isolated more than two decades ago from Drosophila, Aurora A is a widespread protein kinase that plays key roles during cell division. Numerous studies have described the localisation of Aurora A at centrosomes, the mitotic spindle, and, more recently, at mitotic centromeres. In this review, we will summarise the cytoskeletal rearrangements regulated by Aurora A during cell division. We will also discuss the recent discoveries showing that Aurora A also controls not only the dynamics of the cortical proteins but also regulates the centromeric proteins, revealing new roles for this kinase during cell division. Keywords: Aurora A protein kinase, centrosome, mitotic spindle, polarity, centromere, kinetochore, cohesion, transcription 1. Introduction Numerous mitotic events are controlled by phosphorylation signalling pathways. In general, extensive phosphorylation events orchestrate the entry into mitosis, whereas waves of dephosphorylation mark the exit [1]. The Aurora kinases are a family of serine/threonine kinases involved in cell-cycle progression, mostly during the G2 and M phases. The founding member of the family, increase in ploidy (IpI1), was isolated three decades ago from budding yeast through a genetic screen to find mutants required for chromosome segregation [2].