University of Nevada, Reno Responses of Above- And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plant Species of Special Concern and Vascular Plant Flora of the National
Plant Species of Special Concern and Vascular Plant Flora of the National Elk Refuge Prepared for the US Fish and Wildlife Service National Elk Refuge By Walter Fertig Wyoming Natural Diversity Database The Nature Conservancy 1604 Grand Avenue Laramie, WY 82070 February 28, 1998 Acknowledgements I would like to thank the following individuals for their assistance with this project: Jim Ozenberger, ecologist with the Jackson Ranger District of Bridger-Teton National Forest, for guiding me in his canoe on Flat Creek and for providing aerial photographs and lodging; Jennifer Whipple, Yellowstone National Park botanist, for field assistance and help with field identification of rare Carex species; Dr. David Cooper of Colorado State University, for sharing field information from his 1994 studies; Dr. Ron Hartman and Ernie Nelson of the Rocky Mountain Herbarium, for providing access to unmounted collections by Michele Potkin and others from the National Elk Refuge; Dr. Anton Reznicek of the University of Michigan, for confirming the identification of several problematic Carex specimens; Dr. Robert Dorn for confirming the identification of several vegetative Salix specimens; and lastly Bruce Smith and the staff of the National Elk Refuge for providing funding and logistical support and for allowing me free rein to roam the refuge for plants. 2 Table of Contents Page Introduction . 6 Study Area . 6 Methods . 8 Results . 10 Vascular Plant Flora of the National Elk Refuge . 10 Plant Species of Special Concern . 10 Species Summaries . 23 Aster borealis . 24 Astragalus terminalis . 26 Carex buxbaumii . 28 Carex parryana var. parryana . 30 Carex sartwellii . 32 Carex scirpoidea var. scirpiformis . -

Checklist of the Vascular Plants of Redwood National Park
Humboldt State University Digital Commons @ Humboldt State University Botanical Studies Open Educational Resources and Data 9-17-2018 Checklist of the Vascular Plants of Redwood National Park James P. Smith Jr Humboldt State University, [email protected] Follow this and additional works at: https://digitalcommons.humboldt.edu/botany_jps Part of the Botany Commons Recommended Citation Smith, James P. Jr, "Checklist of the Vascular Plants of Redwood National Park" (2018). Botanical Studies. 85. https://digitalcommons.humboldt.edu/botany_jps/85 This Flora of Northwest California-Checklists of Local Sites is brought to you for free and open access by the Open Educational Resources and Data at Digital Commons @ Humboldt State University. It has been accepted for inclusion in Botanical Studies by an authorized administrator of Digital Commons @ Humboldt State University. For more information, please contact [email protected]. A CHECKLIST OF THE VASCULAR PLANTS OF THE REDWOOD NATIONAL & STATE PARKS James P. Smith, Jr. Professor Emeritus of Botany Department of Biological Sciences Humboldt State Univerity Arcata, California 14 September 2018 The Redwood National and State Parks are located in Del Norte and Humboldt counties in coastal northwestern California. The national park was F E R N S established in 1968. In 1994, a cooperative agreement with the California Department of Parks and Recreation added Del Norte Coast, Prairie Creek, Athyriaceae – Lady Fern Family and Jedediah Smith Redwoods state parks to form a single administrative Athyrium filix-femina var. cyclosporum • northwestern lady fern unit. Together they comprise about 133,000 acres (540 km2), including 37 miles of coast line. Almost half of the remaining old growth redwood forests Blechnaceae – Deer Fern Family are protected in these four parks. -

Above Treeline Shrub-Chronologies on the Eastern Sierra Nevada Crest
Above-treeline Linanthus pungens shrub-chronologies on the eastern Sierra Nevada crest, Mono Co., California contain records of precipitation and temperature Rebecca Franklin, Laboratory of Tree-Ring Research, University of Arizona, Tucson, AZ, USA 1. Site Characteristics and Study Description: 4. Barney Lake Chronology Description 6. Climate-Growth Response 10 mi. Initial evaluation of climate- Barney Lake Linanthus pungens A. BLRG Residual EPS, SSS value, .85 cut-off and sample depth growth response. Marker years 1 100 1.4 N chronology in the Barney Lake chronology 0.9 0.8 80 indicate extreme growth Here I present the first shrub-ring chronology 0.7 responses to drought years (1934, 1.2 1934: drought for L. pungens in North America. In panel B is 0.6 60 1961, 1977) and to years with 0.5 the L. pungens residual index values in black BLRG res SSS record snowfall (1983, 1986, 0.4 40 with the +/- 1 SD levels shaded in gray. EPS 0.85 cutoff 1995) –as indicated in the graph 1 0.3 Woody shrub stems and taproots have values for the Barney Lake site chronology BLRG res EPS to the right. 0.2 20 been used in an increasing number of reach 0.85 (for a sample depth of 16) only after BLRG sample depth 0.1 0.8 climatological and ecological applications: 1952 so I truncate the chronology there and Wide annual rings occur during reconstructing glacial fluctuations in 2 0 use only 1952-2007 index values in my 1940 1950 1960 1970 1980 1990 2000 drought years. -

Phlox Douglasii Hook.)
PLANT OF THE YEAR Columbia Phlox (Phlox douglasii Hook.) James H. Locklear 7431 Briarhurst Circle, Lincoln, NE 68506 Phlox douglasii is a subshrub, branching from a woody base with herbaceous growth that dies back to the woody tissue at the end of each growing season. Flowers are borne at the top of the new growth. Photo by James Locklear. hlox douglasii is a name covering…a multitude of in 1820, and helped develop the Glasgow Botanic Garden botanical sins.” So wrote Ira Gabrielson in his 1932 where his path crossed that of a newly hired gardener (David classic, Western American Alpines, and so I discovered Douglas). Hooker was so impressed with the young Scot that for“P myself some 70 years later. With grants from the Native he recommended Douglas to the Royal Horticultural Society of Plant Society of Oregon and the North American Rock Garden London as a botanical collector (Hooker 1836). Douglas made Society, I waded into a study of the genus Phlox in general and his first collecting trip under the auspices of the Society in 1823, P. douglasii in particular. While matters of nomenclature can be traveling to the northeastern United States and Canada. In 1824 he tedious to work through, the species in question is a prominent set sail for the west coast of North America, arriving at the mouth wildflower in a number of plant communities in central and of the Columbia River in April of 1825. In this vast watershed, eastern Oregon, and a clear picture of its taxonomic identity is Douglas collected seeds and plant specimens for the Society, and important to understanding and describing the ecology of these in the process, discovered scores of new species that today bear communities. -
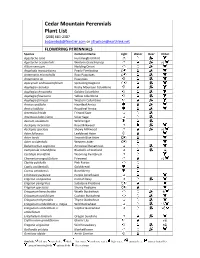
Species List
Cedar Mountain Perennials Plant List (208) 683-2387 [email protected] or [email protected] FLOWERING PERENNIALS Species Common Name Light Water Deer Other Agastache cana Hummingbird Mint Agastache occidentalis Western Giant Hyssop Allium cernuum Nodding Onion Anaphalis margaritacea Pearly Everlasting Antennaria microphylla Rosy Pussytoes Antennaria sp. Pussytoes Apocynum androsaemifolium Spreading Dogbane Aquilegia caerulea Rocky Mountain Columbine Aquilegia chrysantha Golden Columbine Aquilegia flavescens Yellow Columbine Aquilegia formosa Western Columbine Arnica cordifolia Heartleaf Arnica Arnica latifolia Broadleaf Arnica Artemisia frigida Fringed Sage Artemisia ludoviciana Silver Sage Asarum caudatum Wild Ginger Asclepias incarnata Rose Milkweed Asclepias speciosa Showy Milkweed Aster foliaceus Leafybract Aster Aster laevis Smooth Blue Aster Aster occidentalis Western Aster Balsamorhiza sagittata Arrowleaf Balsamroot Campanula rotundifolia Bluebells of Scotland Castilleja linariifolia Wyoming Paintbrush Chamerion angustifolium Fireweed Clarkia pulchella Pink Fairies Coptis occidentalis Goldthread Cornus canadensis Bunchberry Echinacea purpurea Purple Coneflower Erigeron compositus Cutleaf Daisy Erigeron peregrinus Subalpine Fleabane Erigeron speciosus Showy Fleabane Eriogonum heracleoides Wyeth Buckwheat Eriogonum ovalifolium Cushion Buckwheat Eriogonum thymoides Thyme Buckwheat Eriogonum umbellatum v. majus Wild Buckwheat Eriogonum umbellatum v. Sulphur Buckwheat umbellatum Eriophyllum lanatum Oregon Sunshine Erythronium -

Emergent Flowering Plants, P-R – Pg.1
Starflower Image Herbarium & Landscaping Pages Emergent Flowering Plants, P-R – pg.1 Starflower Image Herbarium Emergent Flowering Plants, P-R © Starflower Foundation, 1996-2007 Washington Native Plant Society These species pages has been valuable and loved for over a decade by WNPS members and the PNW plant community. Untouched since 2007, these pages have been archived for your reference. They contain valuable identifiable traits, landscaping information, and ethnobotanical uses. Species names and data will not be updated. To view updated taxonomical information, visit the UW Burke Herbarium Image Collection website at http://biology.burke.washington.edu/herbarium/imagecollection.php. For other useful plant information, visit the Native Plants Directory at www.wnps.org. Compiled September 1, 2018 Starflower Image Herbarium & Landscaping Pages Emergent Flowering Plants, P-R – pg.2 Contents About Ann Lennartz ................................................................................................................................................................ 3 Penstemon serrulatus............................................................................................................................................................. 4 Coast Penstemon ................................................................................................................................................................ 4 Petasites palmatus ................................................................................................................................................................ -

Plant List Lomatium Mohavense Mojave Parsley 3 3 Lomatium Nevadense Nevada Parsley 3 Var
Scientific Name Common Name Fossil Falls Alabama Hills Mazourka Canyon Div. & Oak Creeks White Mountains Fish Slough Rock Creek McGee Creek Parker Bench East Mono Basin Tioga Pass Bodie Hills Cicuta douglasii poison parsnip 3 3 3 Cymopterus cinerarius alpine cymopterus 3 Cymopterus terebinthinus var. terebinth pteryxia 3 3 petraeus Ligusticum grayi Gray’s lovage 3 Lomatium dissectum fern-leaf 3 3 3 3 var. multifidum lomatium Lomatium foeniculaceum ssp. desert biscuitroot 3 fimbriatum Plant List Lomatium mohavense Mojave parsley 3 3 Lomatium nevadense Nevada parsley 3 var. nevadense Lomatium rigidum prickly parsley 3 Taxonomy and nomenclature in this species list are based on Lomatium torreyi Sierra biscuitroot 3 western sweet- the Jepson Manual Online as of February 2011. Changes in Osmorhiza occidentalis 3 3 ADOXACEAE–ASTERACEAE cicely taxonomy and nomenclature are ongoing. Some site lists are Perideridia bolanderi Bolander’s 3 3 more complete than others; all of them should be considered a ssp. bolanderi yampah Lemmon’s work in progress. Species not native to California are designated Perideridia lemmonii 3 yampah with an asterisk (*). Please visit the Inyo National Forest and Perideridia parishii ssp. Parish’s yampah 3 3 Bureau of Land Management Bishop Resource Area websites latifolia for periodic updates. Podistera nevadensis Sierra podistera 3 Sphenosciadium ranger’s buttons 3 3 3 3 3 capitellatum APOCYNACEAE Dogbane Apocynum spreading 3 3 androsaemifolium dogbane Scientific Name Common Name Fossil Falls Alabama Hills Mazourka Canyon Div. & Oak Creeks White Mountains Fish Slough Rock Creek McGee Creek Parker Bench East Mono Basin Tioga Pass Bodie Hills Apocynum cannabinum hemp 3 3 ADOXACEAE Muskroot Humboldt Asclepias cryptoceras 3 Sambucus nigra ssp. -

Washington Park Arboretum (2004) (Pdf)
i Foreword The results of this project are from the combined efforts of a team of talented students at the University of Washington. The five members of this team came to this project with diverse experiences and knowledge. The team included Landscape Architecture students and Environmental Horticulture/ Urban Forestry students; including undergraduate and graduate students. Amongst the team were individuals with extensive experience in nursery management, municipal arboriculture, landscape design, and environmental consulting. The team even included an International Society of Arboriculture Certified Arborist and Washington State University Master Gardeners. We hope that this project will contribute significantly to the implementation of a Native Plant Synoptic Garden at Washington Park Arboretum. The information contained in this project should be used in conjunction with other previous projects that have focused on the creation of this garden. By combining all this cumulative information, the final stakeholders and designers at Washington Park Arboretum will be able to synthesize and create this world class garden. ii Contents FOREWORD……………………………………………………………………………………..i INTRODUCTION………………………………………………………………………………..1 Site Location……………………………………………………………………………………..1 Site History……………………………………………………………………………………....1 Project Goals ……………………………………………………………………………………..2 SITE ANALYSIS………………………………………………………………………………...3 Existing Vegetation…………………………………………………………………………….....6 Topography……………………………………………………………………………………....7 Climate…………………………………………………………………………………………..8 -

Plant List Browder Ridge
*Non-native Browder Ridge Plant List as of 7/12/2016 compiled by Tanya Harvey T14S.R6E.S10,11 westerncascades.com FERNS & ALLIES Abies procera Ribes lacustre Athyriaceae Picea engelmannii Ribes lobbii Athyrium filix-femina Pinus contorta var. latifolia Ribes sanguineum Blechnaceae Pinus monticola Ribes viscosissimum Blechnum spicant Pseudotsuga menziesii Rhamnaceae Cystopteridaceae Tsuga heterophylla Ceanothus velutinus Cystopteris fragilis Tsuga mertensiana Rosaceae Gymnocarpium disjunctum Taxaceae Amelanchier alnifolia Dennstaedtiaceae Taxus brevifolia Holodiscus discolor Pteridium aquilinum TREES & SHRUBS: DICOTS Prunus emarginata Dryopteridaceae Adoxaceae Rosa gymnocarpa Polystichum lonchitis Sambucus racemosa Rubus lasiococcus Polystichum munitum Araliaceae Rubus leucodermis Lycopodiaceae Oplopanax horridus Rubus parviflorus Lycopodium clavatum Berberidaceae Rubus spectabilis Polypodiaceae Berberis nervosa Rubus ursinus Polypodium sp. (Mahonia nervosa) Sorbus scopulina Pteridaceae Betulaceae Sorbus sitchensis Adiantum aleuticum Alnus viridis ssp. sinuata (Adiantum pedatum var. aleuticum) (Alnus sinuata) Spiraea splendens (Spiraea densiflora) Aspidotis densa Corylus cornuta var. californica Salicaceae Cheilanthes gracillima Caprifoliaceae Salix sitchensis Symphoricarpos albus Cryptogramma acrostichoides (Cryptogramma crispa) Symphoricarpos mollis Sapindaceae (Symphoricarpos hesperius) Acer circinatum Selaginellaceae Selaginella scopulorum Celastraceae Acer glabrum var. douglasii (Selaginella densa var. scopulorum) Paxistima myrsinites -

Ground Covers Ground Covers
TheThe AmericanAmerican GARDENERGARDENER TheThe MagazineMagazine ofof thethe AAmericanmerican HorticulturalHorticultural SocietySociety September/October 2005 Spring Bulbs Liven Up Beds and Borders Earth-Friendly TTipsips forfor FallFall CleanupCleanup SimplifyingSimplifying thethe Garden:Garden: WorkWork Less,Less, EnjoyEnjoy MoreMore shade-loving ground covers A green lawn does not have to be hazardous to your health. Why risk exposing your family to the potential health risks associated with unnecessary lawn chemical use? NaturaLawn® of America’s environmentally friendly approach creates a green lawn quickly, more naturally, and with fewer weeds. We know a one-size-fits all chemical program is simply not a safe approach. That’s why we customize a formula that’s right for your lawn. Working with nature, not against it, NaturaLawn of America strengthens your lawn’s root system by building the soil to help give you a healthy green lawn that stays that way. Call 800-989-5444 and we’ll show you that our service is as superior as the lawns we create. NaturaLawn of America, the safer way to a healthy lawn. Find out more at www.nl-amer.com. THE LEADER I N ORGANI C- BASED LAWNCARETM © 2005. NaturaLawn of America, Inc. All rights reserved. Each office independently owned and operated. www.nl-amer.com For permission to reproduce this piece, call 800-989-5444. contents Volume 84, Number 5 . September / October 2005 FEATURES DEPARTMENTS 5 NOTES FROM RIVER FARM 6 MEMBERS’ FORUM 8 NEWS FROM AHS River Farm being transformed for the Eastern Performance Trials, HGI offers new volunteer opportunities for Master Gardeners, new children’s gardening book awards debut in Atlanta. -

Evolutionary Shifts Associated with Substrate Endemism in the Western American Flora
Evolutionary Shifts Associated with Substrate Endemism in the Western American Flora By Adam Christopher Schneider A dissertation submitted in partial satisfaction of the Requirements for the degree of Doctor of Philosophy in Integrative Biology in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Bruce Baldwin, Chair Professor Brent Mishler Professor Kip Will Summer 2017 Evolutionary Shifts Associated with Substrate Endemism in the Western American Flora Copyright © 2017 by Adam Christopher Schneider Abstract Evolutionary Shifts Associated with Substrate Endemism in the Western American Flora by Adam Christopher Schneider Doctor of Philosophy in Integrative Biology University of California, Berkeley Professor Bruce G. Baldwin, Chair This study investigated how habitat specialization affects the evolution and ecology of flowering plants. Specifically, a phylogenetic framework was used to investigate how trait evolution, lineage diversification, and biogeography of the western American flora are affected by two forms of substrate endemism: (1) edaphic specialization onto serpentine soils, and (2) host specialization of non-photosynthetic, holoparasitic Orobanchaceae. Previous studies have noted a correlation between presence on serpentine soils and a suite of morphological and physiological traits, one of which is the tendency of several serpentine-tolerant ecotypes to flower earlier than nearby closely related populations not growing on serpentine. A phylogenetically uncorrected ANOVA supports this hypothesis, developed predominantly through previously published comparisons of conspecific or closely related ecotypes. However, comparisons among three models of trait evolution, as well as phylogenetic independent contrasts across 24 independent clades of plants that include serpentine tolerant species in California and with reasonably resolved phylogenies, revealed no significant affect of flowering time in each of these genera. -

Biological Surveys Conducted in the SEIS Analysis Area at Mt
Biological Surveys Conducted in the SEIS Analysis Area at Mt. Spokane State Park During 2010 Pacific Biodiversity Institute Biological Surveys Conducted in the SEIS Analysis Area at Mt. Spokane State Park During 2010 Peter H. Morrison [email protected] and George Wooten [email protected] Pacific Biodiversity Institute P.O. Box 298 Winthrop, Washington 98862 509-996-2490 Recommended Citation Morrison P.H. and G.Wooten. 2010. Biological Surveys Conducted in the SEIS Analysis Area at Mt. Spokane State Park During 2010. Pacific Biodiversity Institute, Winthrop, Washington. 151 pp. Acknowledgements Brad McQuarrie (manager) and many of the staff at Mt. Spokane Ski Area assisted with logistics and transportation. Hans Smith, Asako Yamamuro and Juliet Rhodes all assisted with field work. Susan Snetsinger assisted with database development and analysis. Asako Yamamuro assisted with data entry. Project Funding This project was funded by the Mt. Spokane Ski Area (Mt. Spokane 2000) Table of Contents Introduction ................................................................................................................................................ 1 Vegetation/Habitat Surveys within the SEIS Analysis Area ............................................................... 2 Methods ................................................................................................................................................... 2 Results .....................................................................................................................................................