U.S. V. Jazz Pharmaceuticals, Inc
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

THE SHARED INFLUENCES and CHARACTERISTICS of JAZZ FUSION and PROGRESSIVE ROCK by JOSEPH BLUNK B.M.E., Illinois State University, 2014
COMMON GROUND: THE SHARED INFLUENCES AND CHARACTERISTICS OF JAZZ FUSION AND PROGRESSIVE ROCK by JOSEPH BLUNK B.M.E., Illinois State University, 2014 A thesis submitted to the Faculty of the Graduate School of the University of Colorado in partial fulfillment of the requirement for the degree of Master in Jazz Performance and Pedagogy Department of Music 2020 Abstract Blunk, Joseph Michael (M.M., Jazz Performance and Pedagogy) Common Ground: The Shared Influences and Characteristics of Jazz Fusion and Progressive Rock Thesis directed by Dr. John Gunther In the late 1960s through the 1970s, two new genres of music emerged: jazz fusion and progressive rock. Though typically thought of as two distinct styles, both share common influences and stylistic characteristics. This thesis examines the emergence of both genres, identifies stylistic traits and influences, and analyzes the artistic output of eight different groups: Return to Forever, Mahavishnu Orchestra, Miles Davis’s electric ensembles, Tony Williams Lifetime, Yes, King Crimson, Gentle Giant, and Soft Machine. Through qualitative listenings of each group’s musical output, comparisons between genres or groups focus on instances of one genre crossing over into the other. Though many examples of crossing over are identified, the examples used do not necessitate the creation of a new genre label, nor do they demonstrate the need for both genres to be combined into one. iii Contents Introduction………………………………………………………………………………… 1 Part One: The Emergence of Jazz………………………………………………………….. 3 Part Two: The Emergence of Progressive………………………………………………….. 10 Part Three: Musical Crossings Between Jazz Fusion and Progressive Rock…………….... 16 Part Four: Conclusion, Genre Boundaries and Commonalities……………………………. 40 Bibliography………………………………………………………………………………. -

Jazz and the Cultural Transformation of America in the 1920S
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2003 Jazz and the cultural transformation of America in the 1920s Courtney Patterson Carney Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the History Commons Recommended Citation Carney, Courtney Patterson, "Jazz and the cultural transformation of America in the 1920s" (2003). LSU Doctoral Dissertations. 176. https://digitalcommons.lsu.edu/gradschool_dissertations/176 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. JAZZ AND THE CULTURAL TRANSFORMATION OF AMERICA IN THE 1920S A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of History by Courtney Patterson Carney B.A., Baylor University, 1996 M.A., Louisiana State University, 1998 December 2003 For Big ii ACKNOWLEDGEMENTS The real truth about it is no one gets it right The real truth about it is we’re all supposed to try1 Over the course of the last few years I have been in contact with a long list of people, many of whom have had some impact on this dissertation. At the University of Chicago, Deborah Gillaspie and Ray Gadke helped immensely by guiding me through the Chicago Jazz Archive. -

INTRODUCTION: BLUE NOTES TOWARD a NEW JAZZ DISCOURSE I. Authority and Authenticity in Jazz Historiography Most Books and Article
INTRODUCTION: BLUE NOTES TOWARD A NEW JAZZ DISCOURSE MARK OSTEEN, LOYOLA COLLEGE I. Authority and Authenticity in Jazz Historiography Most books and articles with "jazz" in the title are not simply about music. Instead, their authors generally use jazz music to investigate or promulgate ideas about politics or race (e.g., that jazz exemplifies democratic or American values,* or that jazz epitomizes the history of twentieth-century African Americans); to illustrate a philosophy of art (either a Modernist one or a Romantic one); or to celebrate the music as an expression of broader human traits such as conversa- tion, flexibility, and hybridity (here "improvisation" is generally the touchstone). These explorations of the broader cultural meanings of jazz constitute what is being touted as the New Jazz Studies. This proliferation of the meanings of "jazz" is not a bad thing, and in any case it is probably inevitable, for jazz has been employed as an emblem of every- thing but mere music almost since its inception. As Lawrence Levine demon- strates, in its formative years jazz—with its vitality, its sexual charge, its use of new technologies of reproduction, its sheer noisiness—was for many Americans a symbol of modernity itself (433). It was scandalous, lowdown, classless, obscene, but it was also joyous, irrepressible, and unpretentious. The music was a battlefield on which the forces seeking to preserve European high culture met the upstarts of popular culture who celebrated innovation, speed, and novelty. It 'Crouch writes: "the demands on and respect for the individual in the jazz band put democracy into aesthetic action" (161). -

Jazz Studies* 1
Jazz Studies* 1 complicated terrains of the New Orleans of Bunk Johnson, for example, or JAZZ STUDIES* the Baltimore of Billie Holiday (born in Philadelphia, reared in Baltimore). They explore such artists’ other geographical travels. What did their *Jazz Studies is offered exclusively as a concentration. images, including mistaken conceptions of who they were, tell us about the cultures that mythologized them? The Center for Jazz Studies: Prentis Hall, 4th floor (632 W. 125th Street); 212-851-9270 How did these jazz musicians influence not only musicians but other http://www.columbia.edu/cu/cjs artists of their era and milieu: the poets and novelists, painters and sculptors, photographers and filmmakers, dancers and choreographers Jazz at Columbia: who regularly heard them play and often shared with them a sense of https://mpp.music.columbia.edu/louis-armstrong-jazz-performance- common project? program One thinks of Tito Puente, working with singers and dancers at the Director: Prof. Robert G. O'Meally, 611 Philosophy; 212-851-9270; Palladium; Jackson Pollack dancing to the music as he spun drips of [email protected] paints on canvasses placed on the studio floor; Langston Hughes writing detailed instructions to the musicians he hoped would accompany Director of Jazz Performance: Prof. Christopher Washburne, 619A Dodge; performance of his poetry; Romare Bearden’s beautifully turned stage 212-854-9862; [email protected] and costume designs for Alvin Ailey and Dianne McIntyre, whose improvisatory jazz dance workshop was called Sound in -

Jazz and Radio in the United States: Mediation, Genre, and Patronage
Jazz and Radio in the United States: Mediation, Genre, and Patronage Aaron Joseph Johnson Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2014 © 2014 Aaron Joseph Johnson All rights reserved ABSTRACT Jazz and Radio in the United States: Mediation, Genre, and Patronage Aaron Joseph Johnson This dissertation is a study of jazz on American radio. The dissertation's meta-subjects are mediation, classification, and patronage in the presentation of music via distribution channels capable of reaching widespread audiences. The dissertation also addresses questions of race in the representation of jazz on radio. A central claim of the dissertation is that a given direction in jazz radio programming reflects the ideological, aesthetic, and political imperatives of a given broadcasting entity. I further argue that this ideological deployment of jazz can appear as conservative or progressive programming philosophies, and that these tendencies reflect discursive struggles over the identity of jazz. The first chapter, "Jazz on Noncommercial Radio," describes in some detail the current (circa 2013) taxonomy of American jazz radio. The remaining chapters are case studies of different aspects of jazz radio in the United States. Chapter 2, "Jazz is on the Left End of the Dial," presents considerable detail to the way the music is positioned on specific noncommercial stations. Chapter 3, "Duke Ellington and Radio," uses Ellington's multifaceted radio career (1925-1953) as radio bandleader, radio celebrity, and celebrity DJ to examine the medium's shifting relationship with jazz and black American creative ambition. -

Jazz Photographs by Herman Leonard January 17 - May 4, 2014
IMPROVISATIONS: Jazz Photographs by Herman Leonard January 17 - May 4, 2014 TEACHER PACKET Biography Of Herman Leonard Herman Leonard (1923-2010) is known for his unique and iconic images of jazz musicians. This exhibition features a selection of 15 silver gelatin prints from Leonard’s 63 works in the Kennedy Museum of Art collection. time. in the darkroom and at sittings, working with subjects like Albert Einstein, Harry Truman and Martha Graham. Photographing in nightclubs, Leonard captured the intensity and passion of jazz’s leading history remains his most remarkable achievement. Jazz and Civil Rights jazz music’s popularity in the 20th century helped prevent complete segregation. With the rise of in-home radios and music clubs, jazz music reached beyond African American communities to the homes of Whites and Latinos. As a symptom of the segregated music industry, record companies in the early 1900s produced blues and jazz music called race records. This music was for black audiences by black musicians. When a jazz song sold well in the African American community, white record companies would remake the song with white musicians to sell to white audiences. As the demand for race records grew, black records featuring black musicians were sold to white audiences. In the 1920s, the radio made jazz more accessible to white audiences. Although restricted and sometimes criticized as too “exotic,” music made by African Americans was being heard forces often created frivolous citations to prevent interracial crowds at jazz clubs. Although character in minstrel shows performed by white actors in blackface. importance of jazz in African American’s lives, stating, “It is no wonder that so much of the the modern essayists and scholars wrote of ‘racial identity’ as a problem for a multi-racial community. -

The Avant-Garde in Jazz As Representative of Late 20Th Century American Art Music
THE AVANT-GARDE IN JAZZ AS REPRESENTATIVE OF LATE 20TH CENTURY AMERICAN ART MUSIC By LONGINEU PARSONS A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2017 © 2017 Longineu Parsons To all of these great musicians who opened artistic doors for us to walk through, enjoy and spread peace to the planet. ACKNOWLEDGMENTS I would like to thank my professors at the University of Florida for their help and encouragement in this endeavor. An extra special thanks to my mentor through this process, Dr. Paul Richards, whose forward-thinking approach to music made this possible. Dr. James P. Sain introduced me to new ways to think about composition; Scott Wilson showed me other ways of understanding jazz pedagogy. I also thank my colleagues at Florida A&M University for their encouragement and support of this endeavor, especially Dr. Kawachi Clemons and Professor Lindsey Sarjeant. I am fortunate to be able to call you friends. I also acknowledge my friends, relatives and business partners who helped convince me that I wasn’t insane for going back to school at my age. Above all, I thank my wife Joanna for her unwavering support throughout this process. 4 TABLE OF CONTENTS page ACKNOWLEDGMENTS .................................................................................................. 4 LIST OF EXAMPLES ...................................................................................................... 7 ABSTRACT -

Understanding Music Popular Music in the United States
Popular Music in the United States 8 N. Alan Clark and Thomas Heflin 8.1 OBJECTIVES • Basic knowledge of the history and origins of popular styles • Basic knowledge of representative artists in various popular styles • Ability to recognize representative music from various popular styles • Ability to identify the development of Ragtime, the Blues, Early Jazz, Bebop, Fusion, Rock, and other popular styles as a synthesis of both African and Western European musical practices • Ability to recognize important style traits of Early Jazz, the Blues, Big Band Jazz, Bebop, Cool Jazz, Fusion, Rock, and Country • Ability to identify important historical facts about Early Jazz, the Blues, Big Band Jazz, Bebop, Cool Jazz, Fusion, and Rock music • Ability to recognize important composers of Early Jazz, the Blues, Big Band Jazz, Bebop, Cool Jazz, Fusion, and Rock music 8.2 KEY TERMS • 45’s • Bob Dylan • A Tribe Called Quest • Broadway Musical • Alan Freed • Charles “Buddy” Bolden • Arthur Pryor • Chestnut Valley • Ballads • Children’s Song • BB King • Chuck Berry • Bebop • Contemporary Country • Big Band • Contemporary R&B • Bluegrass • Count Basie • Blues • Country Page | 255 UNDERSTANDING MUSIC POPULAR MUSIC IN THE UNITED STATES • Creole • Protest Song • Curtis Blow • Ragtime • Dance Music • Rap • Dixieland • Ray Charles • Duane Eddy • Rhythm and Blues • Duke Ellington • Richard Rodgers • Earth, Wind & Fire • Ricky Skaggs • Elvis Presley • Robert Johnson • Folk Music • Rock and Roll • Frank Sinatra • Sampling • Fusion • Scott Joplin • George Gershwin • Scratching • Hillbilly Music • Stan Kenton • Honky Tonk Music • Stan Kenton • Improvisation • Stephen Foster • Jelly Roll Morton • Storyville • Joan Baez • Swing • Leonard Bernstein • Syncopated • Louis Armstrong • The Beatles • LPs • Victor Herbert • Michael Bublé • Weather Report • Minstrel Show • Western Swing • Musical Theatre • William Billings • Operetta • WJW Radio • Original Dixieland Jazz Band • Work Songs • Oscar Hammerstein 8.3 INTRODUCTION Popular music is by definition music that is disseminated widely. -

How Bebop Came to Be: the Early History of Modern Jazz" (2013)
Student Publications Student Scholarship 2013 How Bebop Came to Be: The aE rly History of Modern Jazz Colin M. Messinger Gettysburg College Follow this and additional works at: https://cupola.gettysburg.edu/student_scholarship Part of the Cultural History Commons, and the Ethnomusicology Commons Share feedback about the accessibility of this item. Messinger, Colin M., "How Bebop Came to Be: The Early History of Modern Jazz" (2013). Student Publications. 188. https://cupola.gettysburg.edu/student_scholarship/188 This is the author's version of the work. This publication appears in Gettysburg College's institutional repository by permission of the copyright owner for personal use, not for redistribution. Cupola permanent link: https://cupola.gettysburg.edu/student_scholarship/ 188 This open access student research paper is brought to you by The uC pola: Scholarship at Gettysburg College. It has been accepted for inclusion by an authorized administrator of The uC pola. For more information, please contact [email protected]. How Bebop Came to Be: The aE rly History of Modern Jazz Abstract Bebop, despite its rather short lifespan, would become a key influence for every style that came after it. Bebop’s effects on improvisation, group structure, and harmony would be felt throughout jazz for decades to come, and the best known musicians of the bebop era are still regarded as some of the finest jazz musicians to ever take the stage. But the characteristics of bebop can easily be determined from the music itself. [excerpt] Keywords bebop, music history, Jazz, improvisation, Charlie Parker, Kenny Clarke Disciplines Cultural History | Ethnomusicology | Music Comments This paper was written as the final project for FYS 118-2, Why Jazz Matters: The Legacy of Pops, Duke, and Miles, in Fall 2013. -
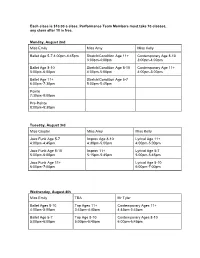
Each Class Is $10.00 a Class. Performance Team Members Must Take 10 Classes, Any Class After 10 Is Free
Each class is $10.00 a class. Performance Team Members must take 10 classes, any class after 10 is free. Monday, August 2nd Miss Emily Miss Amy Miss Kelly Ballet Age 5-7 4:00pm-4:45pm Stretch/Condition Age 11+ Contemporary Age 8-10 3:00pm-4:00pm 3:00pm-4:00pm Ballet Age 8-10 Stretch/Condition Age 8-10 Contemporary Age 11+ 5:00pm-6:00pm 4:00pm-5:00pm 4:00pm-5:00pm Ballet Age 11+ Stretch/Condition Age 5-7 6:00pm-7:30pm 5:00pm-5:45pm Pointe 7:30pm-8:00pm Pre-Pointe 8:00pm-8:30pm Tuesday, August 3rd Miss Crystal Miss Amy Miss Kelly Jazz Funk Age 5-7 Improv Age 8-10 Lyrical Age 11+ 4:00pm-4:45pm 4:30pm-5:00pm 4:00pm-5:00pm Jazz Funk Age 8-10 Improv 11+ Lyrical Age 5-7 5:00pm-6:00pm 5:15pm-5:45pm 5:00pm-5:45pm Jazz Funk Age 11+ Lyrical Age 8-10 6:00pm-7:00pm 6:00pm-7:00pm Wednesday, August 4th Miss Emily TBA Mr Tyler Ballet Ages 8-10 Tap Ages 11+ Contemporary Ages 11+ 4:00pm-5:00pm 3:45pm-4:45pm 4:45pm-5:45pm Ballet Age 5-7 Tap Age 8-10 Contemporary Ages 8-10 5:00pm-6:00pm 5:00pm-5:45pm 6:00pm-6:45pm Ballet 11+ Tap Ages 5-7 6:00pm-7:30pm 6:00pm-6:45pm Pointe 7:30pm-8:00pm Pre-Pointe 8:00pm-8:30pm Thursday, August 5th Miss Amy Miss Kelly Miss Crystal Jazz Ages 5-7 Lyrical Ages 11+ Hip Hop Ages 7-10 4:00pm-4:45pm 4:00pm-5:00pm 7:00pm-8:00pm Jazz Ages 8-10 Lyrical Ages 5-7 Hip hop Age 11+ 5:00pm-6:00pm 5:00pm-5:45pm 8:00pm-9:00pm Jazz Age 11+ Lyrical Ages 8-10 6:00pm-7:00pm 6:00pm-7:00pm Monday, August 9th Miss Emily Miss Crystal Ballet Ages 5-7 Hip Hop Ages 11+ 4:00pm-4:45pm 4:00pm-5:00pm Ballet Ages 8-10 Hip Hop Ages 5-7 5:00pm-6:00pm 5:00pm-5:45pm -

Jazz Style Periods
JAZZ STYLE PERIODS Early Jazz/New Orleans & Chicago Style Dixieland (1920-1930) CHARACTERISTICS: Use of collective improvisation (polyphony). Front line of trumpet (or cornet), clarinet, trombone. New Orleans style typically included banjo and tuba, later replaced by guitar and string bass in Chicago Style. Chicago Style also typically adds saxophone to the front line. Use of flat four in New Orleans Style, later replaced by lighter two beat feel in Chicago Style. Modern drum set emerges when New Orleans musicians begin to consolidate the drum section (bass, snare, cymbals) commonly found in early New Orleans brass bands. IMPORTANT MUSICIANS: Louis Armstrong (cornet/trumpet), Bix Beiderbecke (cornet), Jelly Roll Morton (piano/composer), Sidney Bechet (soprano sax, clarinet), Earl "Fatha" Hines (piano) Swing/Big Band Era (1930-1945) CHARACTERISTICS: Most popular period in jazz history. Large ensembles, less improvisation, more emphasis on written arrangements. Emphasis on showmanship (band uniforms, theme songs, logos on stands, choreography, singers). Development of sections (saxes, trumpets, trombones, rhythm) based on the early model of the front line in New Orleans/Chicago Style Dixieland. Smoother swing feel (steady 4/4 time with emphasis on beats 2 & 4, walking bass, ride cymbal). Features of standard big band arrangements could include: Tutti (all horns playing a melodic line in harmony), Soli (one section featured playing a melodic line in harmony), Shout Chorus (climatic tutti section at the end of the arrangement), Cross-section voicing (a harmonized melodic line voiced using instruments from different sections within the band), Riffs (repeated short melodic and/or rhythmic pattern). IMPORTANT MUSICIANS: Duke Ellington (piano/composer), Count Basie (piano/bandleader), Coleman Hawkins (tenor sax), Lester Young (tenor sax), Roy Eldridge (trumpet) Bop (1945-1950) CHARACTERISTICS: Small ensembles (trio, quartet, quintet). -

University of Alberta 'Indo-Jazz Fusion'
University of Alberta ‘Indo-Jazz Fusion’: Jazz and Karnatak Music in Contact by Tanya Kalmanovitch A thesis submitted to the Faculty of Graduate Studies and Research in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Music Edmonton, Alberta Spring 2008 Abstract An inherently intercultural music, jazz presents a unique entry in the catalog of interactions between Indian music and the west. This dissertation is situated in a line of recent accounts that reappraise the period of Western colonial hegemony in India by tracing a complex continuum of historical and musical events over three centuries. It charts the historical routes by which jazz and Karnatak music have come into contact in the twentieth and twenty-first centuries, both in India and abroad. It presents a detailed account of the history of jazz in India, and Indian music in jazz, and examines jazz’s contact with Karnatak music in three contexts—jazz pedagogy, intercultural collaboration, and the Indian diaspora in the United States—illustrating the musical, social and performative spaces in which these musics come into contact, and describing the specific musical and social practices, and political and social institutions that support this activity. Case studies in this dissertation include an educational exchange between the Jazz and Contemporary Music Program of the New School University (New York, NY) and the Brhaddhvani Research and Training Centre for Musics of the World (Chennai, India), directed by the author in December 2003 – January 2004; a 2001 collaboration between Irish jazz/traditional band Khanda and the Karnataka College of Percussion; and analyses of recent recordings by pianist Vijay Iyer, saxophonist Rudresh Mahanthappa and drummer Ravish Momin.