Reviews/Evaluations Dronabinol Background Claims Data Summary of Clinical Evidence
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tasty THC: Promises and Challenges of Cannabis Edibles
RTI Press Occasional Paper November 2016 Tasty THC: Promises and Challenges of Cannabis Edibles Daniel G. Barrus, Kristen L. Capogrossi, Sheryl C. Cates, Camille K. Gourdet, Nicholas C. Peiper, Scott P. Novak, Timothy W. Lefever, and Jenny L. Wiley RTI Press publication OP-0035-1611 This PDF document was made available from www.rti.org as a public service of RTI International. More information about RTI Press can be found at http://www.rti.org/rtipress. RTI International is an independent, nonprofit research organization dedicated to improving the human condition by turning knowledge into practice. The RTI Press mission is to disseminate information about RTI research, analytic tools, and technical expertise to a national and international audience. RTI Press publications are peer- reviewed by at least two independent substantive experts and one or more Press editors. Suggested Citation Barrus, D.G., Capogrossi, K.L., Cates, S.C., Gourdet, C.K., Peiper, N.C., Novak, S.P., Lefever, T.W., and Wiley, J.L. (2016). Tasty THC: Promises and Challenges of Cannabis Edibles. RTI Press Publication No. OP-0035-1611. Research Triangle Park, NC: RTI Press. http://dx.doi.org /10.3768/rtipress.2016.op.0035.1611 This publication is part of the RTI Press Research Report series. Occasional Papers are scholarly essays on policy, methods, or other topics relevant to RTI areas of research or technical focus. RTI International 3040 East Cornwallis Road PO Box 12194 ©2016 RTI International. All rights reserved. Credit must be provided to the author and source of the Research Triangle Park, NC publication when the content is quoted. -

DRONABINOL What Should You Do If You FORGET a What Other PRECAUTIONS Should Dose? You Follow While Using This Drug?
DRONABINOL What should you do if you FORGET a What other PRECAUTIONS should dose? you follow while using this drug? Other NAMES : Marinol , delta – 9 - If you miss a dose of dronabinol, take it Before starting dronabinol, inform your tetrahydrocannabinol as soon as possible. However, if it is doctor if you have already had in the time for your next dose, do not double past adverse reactions to dronabinol, WHY is this drug prescribed? the dose, just carry on with your regular nabilone (Cesamet ) or marijuana schedule. (pot). Also, notify your doctor if you are Dronabinol is a narcotic drug used to allergic to sesame oil, if you have heart treat severe nausea and vomiting. It is What ADVERSE EFFECTS can this disease (hypertension, abnormal in a family of drugs called cannabinoids drug cause? What should you do heartbeat, angina), or a history of (eg. marijuana). It is also used to about them? emotional disorders such as depression, increase appetite and weight gain. bipolar disease (manic depression), and Dronabinol can cause unsteadiness, schizophrenia (psychosis, HOW should this drug be taken? dizziness, difficulty concentrating, hallucinations). drowsiness, euphoria, abnormal Dronabinol is available in 2.5 mg, 5 mg thinking, fatigue, anxiety, confusion, As this drug may cause some people to and 10 mg capsules. To increase hallucinations, and paranoia. become dizzy, drowsy or less alert than appetite and body weight, the usual Nausea, vomiting, abdominal pain, and normal, you should NOT drive, use initial dose is 2.5 mg twice daily. facial redness may also occur. machinery or do anything else that Depending on the results obtained, your could be dangerous if you are dizzy or doctor might decide to slowly increase These effects may go away during are not alert. -

DRONABINOL- Dronabinol Capsule Actavis Pharma, Inc
DRONABINOL- dronabinol capsule Actavis Pharma, Inc. ---------- DRONABINOL Capsules, USP 2.5 mg, 5 mg, 10 mg CIII Rx only DESCRIPTION Dronabinol is a cannabinoid designated chemically as (6aR-trans)-6a,7,8,10a-tetrahydro-6,6,9- trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol. Dronabinol has the following empirical and structural formulas: Dronabinol, the active ingredient in dronabinol capsules, USP, is synthetic delta-9-tetrahydrocannabinol (delta-9-THC). Delta-9-tetrahydrocannabinol is also a naturally occurring component of Cannabis sativa L. (Marijuana). Dronabinol is a light yellow resinous oil that is sticky at room temperature and hardens upon refrigeration. Dronabinol is insoluble in water and is formulated in sesame oil. It has a pKa of 10.6 and an octanol-water partition coefficient: 6,000:1 at pH 7. Capsules for oral administration: Dronabinol capsules are supplied as round, soft gelatin capsules containing either 2.5 mg, 5 mg, or 10 mg dronabinol. Each dronabinol capsule strength is formulated with the following inactive ingredients: 2.5 mg capsule contains gelatin, glycerin, sesame oil, and titanium dioxide; 5 mg capsule contains iron oxide red and iron oxide black, gelatin, glycerin, sesame oil, and titanium dioxide; 10 mg capsule contains iron oxide red and iron oxide yellow, gelatin, glycerin, sesame oil, and titanium dioxide. CLINICAL PHARMACOLOGY Dronabinol is an orally active cannabinoid which, like other cannabinoids, has complex effects on the central nervous system (CNS), including central sympathomimetic activity. Cannabinoid receptors have been discovered in neural tissues. These receptors may play a role in mediating the effects of dronabinol and other cannabinoids. -

The Rise and Decline of Cannabis Prohibition the History of Cannabis in the UN Drug Control System and Options for Reform
TRANSNATIONAL I N S T I T U T E THE RISE AND DECLINE OF CANNABIS PROHIBITION THE HISTORY OF CANNABIS IN THE UN DruG CONTROL SYSTEM AND OPTIONS FOR REFORM 3 The Rise and Decline of Cannabis Prohibition Authors Dave Bewley-Taylor Tom Blickman Martin Jelsma Copy editor David Aronson Design Guido Jelsma www.guidojelsma.nl Photo credits Hash Marihuana & Hemp Museum, Amsterdam/ Barcelona Floris Leeuwenberg Pien Metaal UNOG Library/League of Nations Archives UN Photo Printing Jubels, Amsterdam Contact Transnational Institute (TNI) De Wittenstraat 25 1052 AK Amsterdam Netherlands Tel: +31-(0)20-6626608 Fax: +31-(0)20-6757176 [email protected] www.tni.org/drugs www.undrugcontrol.info www.druglawreform.info Global Drug Policy Observatory (GDPO) Research Institute for Arts and Humanities Rooms 201-202 James Callaghan Building Swansea University Financial contributions Singleton Park, Swansea SA2 8PP Tel: +44-(0)1792-604293 This report has been produced with the financial www.swansea.ac.uk/gdpo assistance of the Hash Marihuana & Hemp Museum, twitter: @gdpo_swan Amsterdam/Barcelona, the Open Society Foundations and the Drug Prevention and Information Programme This is an Open Access publication distributed under (DPIP) of the European Union. the terms of the Creative Commons Attribution License The contents of this publication are the sole responsibility (http://creativecommons.org/licenses/by/2.0), which of TNI and GDPO and can under no circumstances be permits unrestricted use, distribution, and reproduction regarded as reflecting the position of the donors. in any medium, provided the original work is properly cited. TNI would appreciate receiving a copy of the text in which this document is used or cited. -
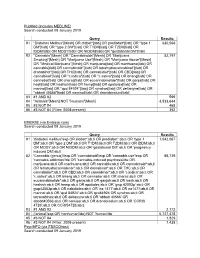
Diabetes Mellitus
PubMed (includes MEDLINE) Search conducted 09 January 2019 Query Results #1 "Diabetes Mellitus"[Mesh] OR diabet*[tiab] OR prediabet*[tiab] OR "type 1 630,566 DM"[tiab] OR "type 2 DM"[tiab] OR T1DM[tiab] OR T2DM[tiab] OR IDDM[tiab] OR MODY[tiab] OR NIDDM[tiab] OR "gestational DM"[tiab] #2 "Cannabis"[Mesh] OR "Cannabinoids"[Mesh] OR "Marijuana 52,197 Smoking"[Mesh] OR "Marijuana Use"[Mesh] OR "Marijuana Abuse"[Mesh] OR "Medical Marijuana"[Mesh] OR marijuana[tiab] OR marihuana[tiab] OR cannabis[tiab] OR cannabinoid*[tiab] OR tetrahydrocannabinol*[tiab] OR dronabinol*[tiab] OR THC[tiab] OR cannabidiol*[tiab] OR CBD[tiab] OR cannabinol*[tiab] OR "c.indica"[tiab] OR "c.sativa"[tiab] OR bhang[tiab] OR cannador[tiab] OR charas[tiab] OR eucannabinolide*[tiab] OR ganja[tiab] OR hash[tiab] OR hashish[tiab] OR hemp[tiab] OR epidiolex[tiab] OR marinol[tiab] OR "qcd 84924"[tiab] OR syndros[tiab] OR deltanyne[tiab] OR "abbott 40566"[tiab] OR namisol[tiab] OR dronabinolum[tiab] #3 #1 AND #2 599 #4 "Animals"[Mesh] NOT "Humans"[Mesh] 4,533,644 #5 #3 NOT #4 468 #6 #3 NOT #4 (Filter: 2008-present) 352 EMBASE (via Embase.com) Search conducted 09 January 2019 Query Results #1 'diabetes mellitus'/exp OR diabet*:ab,ti OR prediabet*:ab,ti OR 'type 1 1,042,067 DM':ab,ti OR 'type 2 DM':ab,ti OR T1DM:ab,ti OR T2DM:ab,ti OR IDDM:ab,ti OR MODY:ab,ti OR NIDDM:ab,ti OR 'gestational DM':ab,ti OR 'pregnancy induced DM':ab,ti #2 'Cannabis (genus)'/exp OR 'cannabinoid'/exp OR 'cannabis use'/exp OR 88,739 'cannabis addiction'/de OR 'cannabis-induced psychosis'/de OR marijuana:ab,ti -

Marijuana in Oregon: a Primer for Clinicians
Legalized Marijuana in Oregon: A Primer for Clinicians Katrina Hedberg, MD, MPH Health Officer & State Epidemiologist History • Originated Central Asia • 3000 BC: Chinese Emperor Shen Nung • Ancient Greeks and Romans familiar with cannabis • 1840’s: Dr. WB O’Shaughnessy, a surgeon working for the British East India Company • 1800’s: Hemp grown in US by President Washington • 1937: US marijuana tax law making it illegal Oregon Public Health Division 2 Marijuana: US Legal Status US DEA Controlled Substances Act Schedule 1 • High potential for abuse • No currently accepted medical use in US (federal level); clinical trials lacking 2018: State Laws • 30 States Medical MJ • 9 recreational Oregon Public Health Division 3 Oregon Medical Marijuana Act • December 1998: Oregon voters approved Ballot Measure 67 • Allows medical use of marijuana (for specific conditions) • Established a state-controlled permit system (the OMMP) • Protects patients and doctors from penalties Oregon Public Health Division 4 Marijuana Sales in Oregon • Jan 2014: Oregon Legislature approved Medical Marijuana Dispensaries (OHA regulates) • July 2015: recreational marijuana became legal • Oct 2015: early sales of retail marijuana allowed at medical dispensaries • Oct 2016: retail marijuana stores open (OLCC regulates) Oregon Public Health Division 5 Objectives • Be familiar with cannabis, cannabinoids, and medical conditions for which scientific evidence supports effectiveness. • Describe elements of Oregon’s clinical guidelines for physicians when recommending use of medical -

5. Cannabis and Cannabis-Related Substances
Annex 1- Extract from the Report of the 41st Expert Committee on Drug Dependence: Cannabis and cannabis-related substances 5. Cannabis and cannabis-related substances 5.1 Cannabis and cannabis resin In the 1961 Single Convention on Narcotic Drugs, cannabis and cannabis resin are described, respectively, as the flowering or fruiting tops of the cannabis plant (excluding the seeds and leaves when not accompanied by the tops) from which the resin has not been extracted and as the separated resin, whether crude or purified, obtained from the cannabis plant. Reference to cannabis below will be taken to also include cannabis resin. Of the many compounds in cannabis, delta-9- tetrahydrocannabinol (Δ9-THC) is the principal psychoactive constituent of cannabis, while cannabidiol (CBD) is also present but is not psychoactive. Following consumption of cannabis, the adverse effects experienced include dizziness and impairment of motor control and cognitive function. As a result of the effects on movement and cognition, cannabis use can impair driving. There are particular risks of cannabis use reported for children, such as respiratory depression, tachycardia and coma. The adverse effects of cannabis consumption are similar to those produced by Δ9-THC alone. There are also a number of adverse effects associated with long term cannabis use, particularly increased risk of mental health disorders such as anxiety, depression and psychotic illness. Chronic regular cannabis use is particularly problematic for young people because of its effects on the developing brain. Cannabis can cause physical dependence in people who use the drug daily or near daily. This is evidenced by the onset of cannabis withdrawal symptoms that occur upon abstinence; these symptoms include gastrointestinal disturbance, appetite changes, irritability, restlessness and sleep impairment. -

2020 Texas Pain Society
Medical Marijuana Kenneth Finn, MD Springs Rehabilitation, PC kfi[email protected] Endocannabinoid System • CB1 receptor • Primarily localized in the central nervous system • Brain and spinal cord • Multiple neurotransmitter effects • Can be found in the peripheral nervous system Endocannabinoid System • CB2 receptor • Inflammation • Neuropathic pain • Cancer pain CBD Dronabinol THC Opioids and Cannabinoid Signaling • Synergistic systems • Both belong to the rhodopsin subfamily of G-protein coupled receptors • Both, when activated, reduce cellular levels of cyclic adenosine monophosphate (cAMP) by inhibiting adenylyl cyclase https://www.tandfonline.com/doi/full/10.1080/24734306.2017.1392715 Opioids and Cannabinoid Signaling • Both receptors found at presynaptic terminals • Both receptors co-localize in GABA-ergic neurons • Both systems share pharmacologic profiles • Sedation, antinociception, hypotension, hypothermia, decreased intestinal motility, drug-reward reinforcement • Naloxone may have effects on the cannabinoid system in several animal models Terminology • Cannabis-based medication • Registered medicinal cannabis extracts with defined and standardized THC and THC/CBD content should be classified as ‘cannabis‐derived’ or ‘cannabis- based’ medicines. • Examples: Epidiolex®, Sativex ®(natural); dronabinol (semi-synthetic); nabilone (synthetic) • Medical cannabis • Cannabis plants and plant material, for example flowers, marijuana, hashish, buds, leaves or full plant extracts used for medical reasons. • Poorly regulated and poorly tested -

Drug Fact Sheet: Marijuana/Cannabis
Marijuana/Cannabis WHAT IS MARIJUANA? What is its effect on the mind? Marijuana is a mind-altering (psychoactive) When marijuana is smoked, the active ingredient drug, produced by the Cannabis sativa plant. THC passes from the lungs and into the Marijuana has over 480 constituents. THC (delta- bloodstream, which carries the chemical to the 9-tetrahydrocannabinol) is believed to be the main organs throughout the body, including the brain. ingredient that produces the psychoactive effect. In the brain, THC connects to specific sites called cannabinoid receptors on nerve cells and WHAT IS ITS ORIGIN? influences the activity of those cells. Marijuana is grown in the United States, Canada, Many of these receptors are found in the parts Mexico, South America, Caribbean, and Asia. of the brain that influence: It can be cultivated in both outdoor and indoor • Pleasure, memory, thought, concentration, sensory and settings. time perception, and coordinated movement What are common street names? The short-term effects of marijuana include: Common street names include: • Problems with memory and learning, distorted • Aunt Mary, BC Bud, Blunts, Boom, Chronic, Dope, perception, difficulty in thinking and problem-solving, Gangster, Ganja, Grass, Hash, Herb, Hydro, Indo, Joint, and loss of coordination Kif, Mary Jane, Mota, Pot, Reefer, Sinsemilla, Skunk, Smoke, Weed, and Yerba The effect of marijuana on perception and coordination are responsible for serious What does it look like? impairments in learning, associative processes, Marijuana is a dry, shredded green/brown mix and psychomotor behavior (driving abilities). of flowers, stems, seeds, and leaves from the Long term, regular use can lead to physical Cannabis sativa plant. -

Benefits and Harms of Cannabis in Chronic Pain Or Post-Traumatic
Benefits and Harms of Cannabis for Chronic Pain or PTSD Evidence-based Synthesis Program APPENDIX A. SEARCH STRATEGIES Databases/Websites · Ovid Medline · PubMed (non-Medline materials) · Elsevier EMBASE · Ovid PsycINFO · PILOTS Database (PTSD search only) · EBM Reviews (CDSR, DARE, HTA, Cochrane CENTRAL, etc) · Conference Papers Index · Clinicaltrials.gov · International Clinical Trials Registry Platform (WHO ICTRP) · ISRCTN · NIH Reporter · AHRQ Gold · American Cancer Society Database of Studies Search Strategies Ovid MEDLINE(R) and Ovid OLDMEDLINE(R) 1946 to December Week 5 2015, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations January 11, 2016 Date Searched: Tuesday January 12, 2016 # Searches Results 1 medical marijuana/ or cannabis/ or marijuana smoking/ or exp Cannabinoids/ or 18682 Cannabaceae/ 2 (cannabis or canabis or cannabinoid* or cannabidiol* or CBD or cannabacae or marijuana or marihuana or hashish or hash or ganja or ganjah or hemp or bhang or charas or THC or 38570 tetrahydrocannabinol* or tetra-hydrocannabinol* or 9?tetrahydrocannabinol* or DELTA?9?- tetrahydrocannabinol*).tw. 3 1 or 2 41269 4 pain/ or acute pain/ or breakthrough pain/ or mastodynia/ or exp musculoskeletal pain/ or exp back pain/ or chronic pain/ or facial pain/ or headache/ or metatarsalgia/ or neck pain/ 205083 or exp neuralgia/ or exp nociceptive pain/ or pain, intractable/ or pain, referred/ 5 (pain or pains or painful* or migraine* or headache* or neuropath* or neuralgia* or arthriti* 770253 or fibromyalg*).tw. 6 4 or 5 823437 7 3 and 6 2868 8 7 and (humans/ not animals/) 1331 9 7 not (humans/ or animals/) 312 10 8 or 9 1643 11 limit 10 to (case reports or comment or editorial or letter or news) 293 86 Benefits and Harms of Cannabis for Chronic Pain or PTSD Evidence-based Synthesis Program 12 cross-section*.tw. -

Medical Marijuana: a Panacea Or Scourge
Review Article Medical marijuana: A panacea or scourge Surender Kashyap, Kartikeya Kashyap1 Department of Pulmonary Medicine, Kalpana Chawla Government Medical College, Karnal, Haryana, 1Ex‑Intern, Government Medical College and Hospital, Chandigarh, India ABSTRACT Marijuana (Cannabis sativa) has been used for recreational and medical purposes since ages. Marijuana smoking is an evil, which is on the rise with about 180.6 million active users worldwide. The recent legalization of marijuana in Uruguay has generated global interest. The purpose of this short review is to describe the various preparations, uses and adverse effects of medical marijuana. It also deals with the adverse effects of marijuana smoking when used for recreational purposes. ased on the current literature, medical use of marijuana is justified in certain conditions as an alternative therapy. KEY WORDS: Cannabis, marijuana smoking, medical marijuana Address for correspondence: Prof. Surender Kashyap, Department of Pulmonary Medicine, Kalpana Chawla Government Medical College, Karnal - 132 001, Haryana, India. E-mail: [email protected] The Brazilian psychopharmacologist E. A. Carlini has the Food and Drug Administration of US (USFDA) also aptly said, “Very few drugs, if at all, have such a tangled does not permit cannabis for medical use except in a few history as a medicine. In fact, prejudice, superstition, conditions where the synthetic cannabinoids have been emotionalism, and even ideology have managed to lead approved for therapeutic purposes.[3] Cannabis smoking cannabis to ups and downs concerning both its therapeutic has been an underreported, but a distinctly prevalent properties and its toxicological and dependence-inducing evil in our society with a prevalence of 3.9% in the age effects.”[1] group of 15-64 years around the world. -

Clinical Evidence of Magistral Preparations Based on Medicinal Cannabis
pharmaceuticals Review Clinical Evidence of Magistral Preparations Based on Medicinal Cannabis Sara Arias 1, Marta Leon 2, Diego Jaimes 1 and Rosa-Helena Bustos 1,* 1 Evidence-Based Therapeutics Group, Clinical Pharmacology, Universidad de La Sabana, Chía 140013, Colombia; [email protected] (S.A.); [email protected] (D.J.) 2 Pain and Palliative Group, Universidad de La Sabana, Chía 140013, Colombia; [email protected] * Correspondence: [email protected]; Tel.: +57-1-8615555 Abstract: Cannabis has been widely used as a medicinal plant for millennia; however, studies related to its main components were first conducted in 1960. Subsequently, laboratories have produced new components and structures related to its active biological properties. Countries that have approved the medicinal use of cannabis impose regulations that govern its clinical and scientific use. One means of administering medicinal cannabis is via a magistral preparation that must have a medical prescription and be prepared in an establishment that meets quality standards to ensure the quantities of its main components, such as tetrahydrocannabinol (THC) and cannabidiol (CBD). Furthermore, suppliers must have a clear indication of its use in the patient before prescription. This review shows the published evidence regarding the clinical use of medicinal cannabis magistral preparations in the management of post-chemotherapy nausea and vomiting, neuropathic pain in multiple sclerosis, and anorexia and cachexia in patients with HIV. Keywords: magistral preparation; medicinal cannabis; anorexia; cachexia in HIV; post-chemotherapy nausea and vomiting; neuropathic pain in multiple sclerosis; pharmacology Citation: Arias, S.; Leon, M.; Jaimes, D.; Bustos, R.-H. Clinical Evidence of Magistral Preparations Based on Medicinal Cannabis.